Abstract
The relationship of weight change has extended to accelerated ageing, yet little is known about the association between weight change and anti-aging protein α-Klotho. This study included 10,972 subjects from the National Health and Nutrition Examination Survey 2007–2016. Participants were measured body weight and height at baseline and recalled weight at young adulthood and middle adulthood. α-Klotho concentrations were quantified. Generalized linear regression models were used to assess the association between weight change and α-Klotho. Across adulthood, maximal overweight, non-obese to obese, and stable obesity were consistently associated with lower serum Klotho levels. Compared with participants who remained at normal weight, from middle to late adulthood, participants experiencing maximal overweight, moving from the non-obese to obese, and maintaining obesity had 27.97 (95% CI: − 46.57 to − 9.36), 39.16 (95% CI: − 61.15 to − 17.18), and 34.55 (95% CI: − 55.73 to − 13.37) pg/ml lower α-Klotho, respectively; similarly, from young to late adulthood, those had 29.21 (95% CI: − 47.00 to − 11.42) , 34.14 (95% CI: − 52.88 to − 15.40), and 36.61 (95% CI: − 65.01 to − 8.21) lower, respectively. Interestingly, from middle to late adulthood, the absolute weight change values of 590 participants who changed from obese to non-obese were negatively associated with serum α-Klotho. Each 1 kg of weight loss during the process of changing from obese to non-obese brought about a relative increase in α-Klotho levels of 3.03 pg/ml. The findings suggest the potential role of weight management across adulthood for aging.
Similar content being viewed by others
Introduction
Klotho protein is a anti-aging protein encoded by the Klotho gene, three members of the Klotho family have been identified: α-, β-, and γ-Klotho, which are all single-pass transmembrane proteins1,2. α-Klotho is mostly expressed in the distal convoluted tubules of the kidney and functions as an obligatory coreceptor for fibroblast growth factor 23 (FGF23). After being broken down by secretases, α-Klotho can be released from cells into the circulation3,4. Previous research has discovered that Klotho expression is biologically down-regulated in aged mice5. Conversely, its transgenic over-expression has been shown to increase lifespan in transgenic mice by up to 30%6. α-Klotho has been regarded as an anti-aging biomarker, and α-Klotho deficiency has been associated with a variety of aging-related illnesses, such as cardiovascular disease (CVD)7,8, cancer9, chronic kidney disease10,11, type 2 diabetes mellitus12 and osteoporosis13. Klotho is primarily expressed in kidney and brain tissues and has hormone-like functions. Its anti-aging mechanism is associated with the down-regulation of phosphate reabsorption and the up-regulation of calcium reabsorption, which inhibits the increase of vitamin D levels. Another mechanism involves inhibiting various Wnt ligands, which are linked to aging and tumorigenesis. Additionally, the protein may have an important role in anti-aging through its anti-inflammatory effect14,15,16. Indeed, it has been established that α-Klotho was negatively correlated with age in humans. With age, progressive declines in muscle mass, quality, and function lead to sarcopenia. Considering that α-Klotho is involved in several key processes that regulate skeletal muscle function (e.g. muscle regeneration, mitochondrial biogenesis, endothelial function, oxidative stress, and inflammation), the potential Klotho-based therapeutic interventions will treat sarcopenia and hinder its progression to physical disability17. Unlike other aging indicators, the concentration of α-Klotho in the body can be changed through peripheral administration and other methods. Studies have shown that systemic low-dose Klotho treatment may have a therapeutic effect on the elderly18.
In the US, the prevalence of obesity in adults increased nearly threefold from 1975 to 2016, from 11 to 36% for men and 14% to 38% for women19. Weight change is unavoidable for adults across the lifespan and has been shown to be associated with adverse age-related health outcomes, such as cardiovascular disease, diabetes, cancer and mortality20,21,22,23. While previous studies have included single-point Body weight changes (BMI) and ignored dynamic changes in body weight over time, more recent studies have focused on the long-term consequences of weight changes across adulthood. Recently, the correlation of weight change also has extended to biological aging. A study of Chinese women aged 40–70 found that participants who maintained normal weight or lost weight to normal range since age 50 had a longer mean of telomere length than those who retained obesity or progressed from non-obesity to obesity24. In a separate study using data from 647 women aged 35 to 74 in the United States and Puerto Rico found that weight gain since the age of 30 and weight cycling were inversely linked to telomere length25. The National Health and Nutrition Examination Survey (NHANES) database shows weight gain during a 10-year period promotes aging across adulthood. Specifically, with stable normal weight as a reference group, people with stable obesity, weight gain from non-obesity to obesity, normal weight to overweight or remaining overweight had a shorter leukocyte telomere26. Prior research has primarily focused on evaluating weight change from middle to late adulthood and has been restricted to female participants. A recent study utilizing data from 5553 adults in the NHANES used Phenotype Age Acceleration as a calculated indicator of biological aging. The study found that maximal overweight, non-obese to obese, and stable obesity across adulthood, as well as an obese to non-obese pattern from middle to late adulthood, were associated with accelerated biological aging27.
To the best of our knowledge, no research has been conducted to study the relationship between weight change across adulthood and α-Klotho levels in a nationally representative population. In this study, we leveraged data of 10,972 adults aged ≥ 40 years from the NHANES. We aimed to estimate the associations between weight changes in two stages of lifespan (i.e., in 10 year period before baseline and from age 25 years to the baseline) and α-Klotho.
Materials and methods
Study population
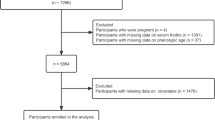
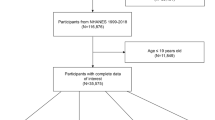
The NHANES used a complex, multistage, probability sampling method to collect nationally representative health-related data on the US population28. The NHANES was approved by the NCHS Research Ethics Review Board, and all participants signed the informed written consent29. In this study, a total of 50,588 subjects from NHANES 2007–2016 cycle were enrolled. We incorporated 19,344 participants aged 40 or over and sequentially excluded pregnant women (n = 14), self-reported cancer patients (n = 2590), subjects with energy intake less than 500 kcal or more than 5000 kcal (n = 274), individuals with missing data for BMI at baseline (n = 851), or recalled body weight at both the age of 25 y (n = 564) and 10 y before recruitment (n = 680), or α-Klotho (n = 3399). Finally, a total of 10,972 participants were eligible for further analyses (Fig. 1).
Assessment of weight change
The participants’ baseline height and weight were measured during a physical examination. The weights of 25 years old and 10 years before the survey were recalled by respondents. BMI at age 25 years (BMI25), at 10 years before baseline survey (BMI10prior) and at baseline survey (BMIbaseline) were calculated as the corresponding weight (kg) divided by the square of baseline height (m2). We created weight change patterns for two time intervals: BMI10prior to BMIbaseline and BMI25 to BMIbaseline, and defined five weight change patterns by using BMI at two time points (supplementary Table S1): stable normal pattern (< 25.0 at both times), maximum overweight pattern (25.0–29.9 at either time but not ≥ 30.0 at the other time), obese to non-obese pattern (≥ 30.0 at younger age and < 30.0 later), non-obese to obese pattern (< 30.0 at younger age and ≥ 30.0 later), and stable obesity (≥ 30.0 at both times). The method has been thoroughly documented in prior articles using NHANES data20,30. We also classified absolute weight change in each time interval into five groups: weight loss of at least 2.5 kg, weight change within 2.5 kg (reference group), weight gain of at least 2.5 kg but less than 10.0 kg, weight gain of at least 10 kg but less than 20.0 kg, and weight gain of at least 20.0 kg31.
Measurement of serum α-Klotho
Serum samples were collected from participants, and then they were stored at − 80 °C. They were shipped on dry ice to the Northwest Lipid Metabolism and Diabetes Research Laboratories at University of Washington in Seattle, Washington in 2019–2020. Serum α-Klotho concentrations were performed using an ELISA kit manufactured by IBL International in Japan. All study samples were analyzed twice, with the result being the average of the two concentrations. The sensitivity of the test was 6 pg/mL. A completed description of the laboratory methodology can be found at https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSKL_H.htm.
Assessment of covariates
On the basis of a literature review, we selected several potential confounders as covariates. The covariates can be classified into demographic variables, lifestyle variables and medical comorbidities. The demographic variables included baseline age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), educational level (less than high school, high school or equivalent and college or above), marital status (married, separated, never married), and family income-to-poverty ratio (PIR) (0–1.0, 1.1–3.0 and > 3.0). Lifestyle factors included healthy eating index score 2015 (divided into four equal groups), energy intake, alcohol drinking (never, former, low to moderate drinker defined as drinking < 1 drink/day in women or < 2 drinks/day in men, and heavy drinker defined as ≥ 1 drink/day in women or ≥ 2 drinks/day in men), smoking status (never, former, current smoker) and leisure-time physical activity. Leisure-time physical activity was categorized into inactive (no leisure-time physical activity), insufficiently active ( leisure-time moderate activity 1–5 times per week with MET ranging from 3 to 6 or leisure-time vigorous activity 1–3 times per week with MET > 6), or active (those who had more leisure-time moderate-or-vigorous activity than above). The medical comorbidities included family history of diabetes, family history of heart attack, self-reported health status (excellent, good, poor), self reported baseline history of diabetes, hypertension, chronic kidney disease (CKD), and cardiovascular disease (CVD). diabetes includes self-reported diabetes, taking antidiabetic drugs or insulin, glycosylated hemoglobin (HbA1c) level ≥ 6.5%, fasting plasma glucose level ≥ 7.0 mmol/L, or 2-h plasma glucose level after an oral glucose tolerance test ≥ 11.1 mmol/L. hypertension includes self-reported hypertension, measured systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or self-reported use of antihypertensive medications. If a participant was previously told that he/she had congestive heart failure, coronary heart disease, angina/angina pectoris, heart attack, or stroke, having one or more of these conditions is defined as cardiovascular disease. Chronic kidney disease has been characterized as a glomerular filtration rate of less than 60 mL/min/1.73 m2, or a urinary albumin-creatinine ratio of 30 or higher. In addition, we also included 25(OH)D.
Statistical analyses
We analyzed the baseline characteristics based on weight change patterns in the two intervals. We used the chi-square test for categorical variables and employed variance (ANOVA) and Kruskal–Wallis tests for continuous variables with normal and skewed distributions, respectively. Generalized linear regression analysis were used to investigate the independent association between weight change and serum α-Klotho. We set up five patterns of weight change over two time intervals: change in BMI from age 25 to baseline, which approximates weight change from young to late-adulthood; and change in BMI in 10 years period before baseline, which approximates weight change from mid- to late adulthood. Model 1 was adjusted for age, gender, and race. Model 2 was further adjusted for educational level, family PIR, marital status, physical activity, healthy eating index score, energy intake, alcohol drinking and smoking status. Model 3 included family history of diabetes, family history of angina, self-reported health status, self reported baseline history of CVD, diabetes, hypertension, and CKD, and 25(OH)D in addition to the covariates in Model 2. Percentages of missing values of covariates were less than 5% except for healthy eating index score (5.6%), self-reported health status (6.6%), drinking status (6.9%) and family income-poverty ratio (7.3%). Additionally, smoothing function analysis were utilized to investigate the possible dose–response association between age or absolute weight change and serum α-Klotho levels.
In NHANES, sampling weights are often used to produce representative and unbiased statistics. However, several studies revealed that the application of weights can cause over-adjustment if the variables used to calculate sampling weights (such as race, age, and income) are further adjusted in the regression analysis. Therefore, in our main analysis, we used unweighted models to ensure accurate estimation, as performed in previous studies32.
We did a string of sensitivity analyses to test the robustness of the results. First, we evaluated the effect of absolute weight change on α-Klotho during two time intervals. Second, potential modifications of the relationship between weight change and Klotho were assessed, including the following variables: age, sex, hypertension, diabetes, CVD and CKD. Heterogeneity among subgroups was evaluated using generalized linear models, and interactions between subgroups and weight change were examined using likelihood ratio testing. Third, we excluded underweight participants to reduce the influence of underweight on the results.
All analyses were performed using the statistical program R-4.2.1 (The R Foundation), Free Statistics software version 1.9.133 and Empowerstats software version 2.0. A two-tailed p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The present study was exempt from ethics approval as it involved a secondary analysis of publicly available NHANES data, in accordance with the regulatory framework of the US Health and Human Services (HHS) at 45 CFR 46.101 (b). The US National Center for Health Statistics Research Ethics Review Board (ERB) approved the NHANES surveys.
Results
Characteristics of study participants
In total, 10,972 patients were included the analysis dataset, with a median age of 54.0 (IQR, 47.0–62.0) years, and 48.6% were males. Participants gained 13.4 kg from age 25 years to baseline, and 4.1 kg in the 10 year period before baseline. From 10 year period before baseline to baseline, 17.4% of the participants shifted from non-obesity to obesity and gained 15.8 kg on average, and 5.4% of participants moved from obesity to non-obesity and lost 12.3 kg on average (Table1). From age 25 years to baseline, 21.9% of the participants were classified as the stable normal, and 7.1% were classified as stable obese (supplementary Table S2). The mean baseline concentration of α-Klotho for all participants was 858.0 pg/ml. Maintaining normal weight from middle to late adulthood and from young to late adulthood has the largest mean α-Klotho measurements (881.7 pg/ml and 880.2 pg/ml, respectively); an obese to non-obese weight change pattern from middle to late adulthood is the lowest (832.5 pg/ml), and maximum overweight from young to late adulthood is shown as the least (846.6 pg/ml). Serum α-Klotho decreased with age at a mean rate of 1.89 pg/ml (95%CI: − 2.61 to − 1.16) per year for the general population (supplementary Figure S1).
The characteristics of study participants among the five weight change patterns in 10 years period before baseline are shown in Table 1. Compared to individuals in the stable normal weight group, those in the other groups were more likely to be male, non-Hispanic black or Mexican–American, less educated, financially disadvantaged, physically inactive, former smokers and drinkers, have a higher percentage of family history of diabetes or heart attack, as well as prevalent diabetes, hypertension, cardiovascular disease, or chronic kidney disease. They were less likely to be insufficiently active, in very good to excellent health, to maintain good diet quality, and to have elevated 25(OH)D levels. Participants in the obese to non-obese group tended to be older, whereas those in the non-obese to obese category and the stable normal group tended to be younger, and those in the maximum overweight category were not significantly different from the stable obese group. Participants in the stable obese group and maximum overweight category tended to have higher levels of energy intake, whereas those in the obese to non-obese category tended to be the lowest total energy intake. Participants in the stable normal group and the obese to non-obese category were more likely to be a current smoker, and those in the non-obese to obese category were more likely to be heavy drinker, whereas those in the stable obese group were less likely to be current smokers and heavy drinker. The distributions of participants’ characteristics according to the weight change pattern from 25 years old to baseline are shown in supplementary Table S2, and the distribution patterns were basically similar except for age, energy intake, smoking and alcohol drinking status.
Associations of weight change patterns with serum α-Klotho
Table 2 shows the associations of weight change patterns across adulthood with serum α-Klotho in three models. Compared with participants who remained at normal weight, from middle to late adulthood, participants experiencing maximal overweight, moving from the non-obese to obese, and maintaining obesity had 27.97 (95% CI: − 46.57 to − 9.36, p = 0.003), 39.16 (95% CI: − 61.15 to − 17.18, p < 0.001), and 34.55 (95% CI: − 55.73 to − 13.37, p = 0.001) pg/ml lower α-Klotho, respectively; similarly, from young to late adulthood, those had 29.21 (95% CI: − 47.00 to − 11.42, p = 0.001), 34.14 (95% CI: − 52.88 to − 15.40, p < 0.001), and 36.61 (95% CI: − 65.01 to − 8.21, p = 0.012) lower, respectively. Interestingly, from middle to late adulthood, the absolute weight change values of 590 participants who changed from obese to non-obese showed a linear negative associated with serum α-Klotho (Fig. 2). Each 1 kg of weight loss during the process of changing from obese to non-obese brought about a relative increase in α-Klotho levels of 3.03 pg/mL. The α-Klotho levels in the second tertile (T2, weight loss range 16.21 to 8.86 kg) were 69.67 lower (95% CI: − 141.37 to 2.03) than the reference group (T1, weight loss range 62.51 to 16.24 kg). Similarly, the Klotho levels in the third tertile (T3, weight loss range 8.82 to 0.85 kg) were 124.77 lower (95% CI: − 197.94, − 51.60, p < 0.001) than the reference group (p for trend < 0.001) (Table 3).
The Association of absolute weight change from obese to non-obese with serum α-Klotho in 10 years period before baseline. The solid and dotted lines represent the estimated values and their corresponding 95% CI, respectively. The model was adjusted for age, gender, race, education level, family income-poverty ratio level, marital status, leisure-time physical activity, healthy eating index score, energy, smoking status and alcohol drinking, family history of diabetes, family history of angina, self-reported health status, self reported baseline history of CVD, diabetes, hypertension, and CKD, and 25(OH)D. Only 1–100% of the data is shown.
Sensitivity analysis
First, in our initial analysis, we examined the impact of absolute weight change as a categorical variable on α-Klotho levels. Specifically, our observations revealed that individuals who experienced a weight gain of 2.5 kg to 10 kg in the 10-year period before baseline showed a 19.53 (− 38.43 to − 0.63) lower level of α-Klotho compared to the group with weight loss or a gain of less than 2.5 kg. Additionally, other weight gain ranges also showed lower α-Klotho levels, although these differences did not reach statistical significance (p > 0.05). Furthermore, from 25 years old to baseline, the three patterns of weight gain also show a trend of lower α-Klotho (supplementary Table S3). Second, the forest plot showed that the association between weight change patterns and α-Klotho was consistent across age, gender, and medical comorbidities (CVD, diabetes and hypertension, CKD) subgroups (Supplementary Figure S2, S3). Apart from diabetes, there was no significant interaction between α-Klotho and the stratification variables. Third, when we excluded underweight participants, similar results were observed (supplementary Table S4).
Discussion
In this large cross-sectional study of a nationally representative sample of U.S. adults, we observed a significant impact of weight status on serum α-Klotho levels throughout adulthood. Across five weight change patterns, serum α-Klotho was greatest for those who maintained a normal weight, and maximum overweight, non-obesity to obesity, and stable obesity showed lower levels of serum α-Klotho. In contrast, weight loss from middle to late adulthood was associated with relative increase in α-Klotho levels. The findings of our study underscore the significance of maintaining a normal body weight in decelerating the aging process.
The association between weight change patterns and biological aging has recently been popular among researchers. Zhang et al.'s26 study of US adults, which utilized telomere length as an aging marker, yielded comparable findings. Normal weight to overweight or stay overweight, weight gain from non-obese to obese, and stable obesity from middle to late adulthood were associated with lower telomere lengths. Cao et al.27 observed the relationship between accelerated biological aging and weight change using an indicator of biological aging (i.e., accelerated phenotypic age [accelerated phenotypic aging]), and they similarly concluded that maximal overweight, non-obesity to obesity, and stable obesity from youth to late-adulthood, as well as from mid- to late adulthood, were all associated with accelerated biological aging. Orces's34 revealed a significant negative correlation between obesity and serum α-Klotho levels in women, utilizing data from the NHANES 2013–2016. In contrast, our study encompassed a nationally representative sample comprising both men and women. Additionally, we adjusted for a broader array of confounding factors and bolstered the robustness of our results by conducting sensitivity analyses. These data supported the theory that obesity may accelerate the aging process, possibly due to obesity-related metabolic dysregulation, which closely resembles the processes observed in normal aging, including inflammation, oxidative stress, cellular senescence, and mitochondrial dysfunction35,36.
Katherine et al.37 examined the effect of a behavioral weight loss intervention on serum Klotho concentration in 152 overweight or obese adults. The study revealed a significant disparity in Klotho concentration changes between adults who achieved a weight loss of ≥ 10% and those with weight loss < 5% within 12 months, regardless of exercise. Their research suggests that weight reduction in middle-aged adults with overweight or obesity may have a positive impact on aging-related biomarkers. Consistent with our findings, we observed, using different models, that transitioning from a non-obese to obese status during middle to late adulthood was associated with relatively higher levels of α-Klotho. These findings support the theory that weight loss interventions may contribute to extending lifespan38,39, aligning with our previous assertion that maintaining a healthy body weight provides substantial benefits.
Previous research has shown that α-Klotho levels decrease with age17. Consequently, we conducted a subgroup analysis stratified by age. We categorized the participants into two groups: those under 60 years old and those 60 years old and above. When examining the impact of weight change patterns on α-Klotho, we found no significant interaction. However, the contrast in serum α-Klotho levels among participants transitioning from non-obese to obese, compared with those who maintained a normal weight, was more pronounced in younger individuals during both time periods (non-obese to obese: < 60y β = 42.09 (− 70.35 to − 13.83) vs ≥ 60y β = 30.14 (− 65.49 to 5.21) in 10 years period before baseline; < 60y β = − 36.89 (− 61.79 to − 12.00) vs ≥ 60y β = − 28.10 (− 56.78 to 0.58) from 25 years old to baseline, Supplementary Figure S2, S3). This could be attributed to the fact that older individuals may already have relatively low α-Klotho levels, making further changes less significant. We also observed that the correlation between weight change patterns and α-Klotho is stronger in women compared to men. This could be attributed to gender variations in fat distribution, resulting in a more pronounced correlation between BMI and women's health status40.
Additionally, our discovery revealed that the influence of weight change in the 10 years preceding the baseline on α-Klotho was more pronounced in patients with CKD, diabetes, hypertension, or CVD, although there was no significant interaction (Supplementary Figure S2). Epidemiological research has repeatedly shown that patients with cardiovascular, renal, and metabolic illnesses frequently overlap and coexist with one another41. Recent statistics show that the prevalence of CKD in type 2 diabetes (T2D) patients is close to 40% and the prevalence in heart failure patients is close to 50%42,43. The molecular mechanisms by which diabetes affects the cardiovascular and renal systems mainly include mitochondrial superoxide production, increased oxidative stress, increased reactive oxygen species (ROS) production, activation of protein kinase C (PKC), and advanced glycation end products (AGE)44,45. In addition, hyperglycemia is associated with the activation of the local renin–angiotensin–aldosterone system (RAAS) in the myocardium and kidneys, thereby promoting organ dysfunction46. There are several potential biological explanations for the association between heart failure and kidney disease. In addition to hemodynamic mechanisms such as low cardiac output and increased renal venous pressure, complex neurohormonal changes also affect renal blood flow and glomerular perfusion47. Serum α-Klotho as a circulating substance originating primarily from the kidney is closely related to kidney function48,49, Rotondi et al.50 found a statistically significant correlation between soluble α-Klotho levels and the renal function index estimated glomerular filtration rate (eGFR), which proposes that kidney disease begins to negatively impact circulating s-Klotho early, implying that CKD patients should pay more attention to weight changes. CVD is the leading cause of death in patients with CKD, and disturbances in mineral homeostasis during CKD progression amplify traditional cardiovascular risk factors. FGF23 regulates mineral homeostasis by activating a complex of FGF receptors and the transmembrane Klotho co-receptor. CKD-associated FGF23 excess and Klotho deficiency are novel risk factors for CVD51.
Interesting, we found that participants who changed from obese to non-obese during the 10-year period before baseline had 33.99 (95% CI: − 65.69 to − 2.30) pg/ml lower α-Klotho than those who remained at normal weight during the same period of time. The sample size of participants from obese to non-obese was only 590, which was significantly smaller than that of other groups, which may be the reason for the wide 95% confidence interval. the estimates mean that the serum α-Klotho levels could be 65.69 pg/ml lower in those who changed from obese to non-obese than those who remained at normal weight. This estimate was even larger in value than those of maximum overweight, non-obese to obese, and stable obese groups. This suggests that among the obese to non-obese population, α-Klotho in a minority of people is affected by other factors. As shown in Table 1, the obese to non-obese group was more likely to have CVD (20.9%) and CKD (26.7%) than participants in other weight change groups. We believe that chronic diseases may be potential factors affecting Klotho. This idea was confirmed in our stratified analysis.
This study has several strengths, including its larger sample size, relatively comprehensive covariate information, and the use of sensitivity analyses, which resulted in a more accurate and practical understanding of the relationship between weight change patterns and serum α-Klotho. This study also has a few limitations. First, using BMI as the sole indicator of adiposity is a limitation, as changes in other obesity markers such as body fat and waist circumference were not available in the NHANES data, thus limiting the inferences that can be drawn from BMI alone. Second, Many covariates were only available at baseline, which meant that we were unable to use time-varying covariates to account for changes in potential confounding factors over time, potentially resulting in residual confounding that could have affected weight change and α-Klotho levels. Third, even though regression models and stratified analyses analysis were performed, residual confounding effects from unmeasured or unknown factors could not be excluded entirely. Fourth, due to the cross-sectional nature of the study, we are unable to assess whether changes in α-Klotho levels occur before or after the weight change, and thus cannot determine their causal relationship. Fifth, the use of self-reported weight data in the analysis introduces the potential for recall bias. In addition, considering ethnic differences, it is uncertain whether the findings of this study can be applied to other ethnic groups.
Conclusions
Our findings demonstrate that maximal overweight, non-obese to obese, and stable obesity across adulthood were associated with lower serum α-Klotho. In contrast, weight loss from middle to late adulthood was associated with relative increase in α-Klotho levels. This underscores the significance of preventing weight gain and maintaining a normal weight among adults in the United States. As the aging population continues to grow, monitoring weight fluctuation could assist in identifying individuals at a heightened risk of accelerated aging and, ultimately, delaying aging-related health complications.
Data availability
The datasets used and analyzed in the current study are publicly available on the NHANES websitee (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
References
Hu, M. C. et al. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 75, 503–533 (2013).
Kuro-O, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15(1), 27–44 (2019).
Hu, M. C. et al. Renal production, uptake, and handling of circulating αKlotho. J. Am. Soc. Nephrol. 27(1), 79–90 (2016).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281(10), 6120–6123 (2006).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655), 45–51 (1997).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309(5742), 1829–1833 (2005).
Chen, W. Y. Soluble alpha-klotho alleviates cardiac fibrosis without altering cardiomyocytes renewal. Int. J. Mol. Sci. 21(6), 2186 (2020).
Xu, J. P. et al. Associations between serum soluble α-Klotho and the prevalence of specific cardiovascular disease. Front. Cardiovasc. Med. 9, 899307 (2022).
Mencke, R., Olauson, H. & Hillebrands, J. L. Effects of Klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 121, 85–100 (2017).
Kim, H. J. et al. Serum klotho is inversely associated with metabolic syndrome in chronic kidney disease: Results from the KNOW-CKD study. BMC Nephrol. 20(1), 119 (2019).
Zou, D. et al. The role of klotho in chronic kidney disease. BMC Nephrol. 19(1), 285 (2018).
Zhang, L. & Liu, T. Clinical implication of alterations in serum Klotho levels in patients with type 2 diabetes mellitus and its associated complications. J. Diabetes Complications 32(10), 922–930 (2018).
Ogata, N. et al. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone 31(1), 37–42 (2002).
Tang, X. et al. Klotho: A tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab. Investig. 96(2), 197–205 (2016).
Landry, T., Shookster, D. & Huang, H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism 121, 154819 (2021).
Wang, F. & Zheng, J. Association between serum alpha-Klotho and severe abdominal aortic calcification among civilians in the United States. Nutr. Metab. Cardiovasc. Dis. 32(6), 1485–1492 (2022).
Abraham, C. R. & Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 82, 101766 (2022).
Castner, S. A. et al. Longevity factor klotho enhances cognition in aged nonhuman primates. Nat. Aging 3(8), 931–937 (2023).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390(10113), 2627–2642. https://doi.org/10.1016/S0140-6736(17)32129-3 (2017).
Chen, C. et al. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ 367, l5584 (2019).
Prestgaard, E. et al. Change in cardiorespiratory fitness and risk of stroke and death: Long-term follow-up of healthy middle-aged men. Stroke 50(1), 155–161 (2019).
Teras, L. R. et al. Sustained weight loss and risk of breast cancer in women 50 years and older: A pooled analysis of prospective data. J. Natl. Cancer Inst. 112(9), 929–937 (2020).
Liu, M. et al. Relationship of weight change patterns from young to middle adulthood with incident cardiovascular diseases. J. Clin. Endocrinol. Metab. 106(2), e812–e823 (2021).
Cui, Y. et al. Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity (Silver Spring) 21(12), 2582–2588 (2013).
Kim, S. et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol. Biomarkers Prev. 18(3), 816–820 (2009).
Zhang, Y. et al. Association between weight change and leukocyte telomere length in U.S. adults. Front. Endocrinol. 12, 650988 (2021).
Cao, X. et al. Weight change across adulthood and accelerated biological aging in middle-aged and older adults. Am. J. Clin. Nutr. 117(1), 1–11 (2023).
Cetners for disease control and prevention. About the National health and nutrition examination survey. NHANES. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 8 January 2022
National Center for Health Statistics. Centers for Disease Control and Prevention NCHS research ethics review board (ERB) approval. https://www.cdc.gov/nchs/nhanes/irba98.htm. Accessed 8 January 2022
Stokes, A. et al. Obesity progression between young adulthood and midlife and incident diabetes: A retrospective cohort study of U.S. adults. Diabetes Care 41(5), 1025–1031 (2018).
Zheng, Y. et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 318(3), 255–269 (2017).
Luo, K. et al. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ. Int. 136, 105461 (2020).
Yang, Q. et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: A cohort study. Front. Med. (Lausanne) 8, 640785 (2021).
Orces, C. H. The association of obesity and the antiaging humoral factor klotho in middle-aged and older adults. ScientificWorldJournal 2022, 7274858 (2022).
Santos, A. L. & Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 67, 101268 (2021).
Lin, W. Y. Lifestyle factors and genetic variants on 2 biological age measures: Evidence from 94443 Taiwan biobank participants. J. Gerontol. A Biol. Sci. Med. Sci. 77(6), 1189–1198 (2022).
Collins, K. A. et al. Change in circulating klotho in response to weight loss, with and without exercise, in adults with overweight or obesity. Front. Aging 4, 1213228 (2023).
Petersen, C. L., Christensen, B. C. & Batsis, J. A. Weight management intervention identifies association of decreased DNA methylation age with improved functional age measures in older adults with obesity. Clin. Epigenet. 13(1), 46 (2021).
Mason, A. E. et al. Weight loss maintenance and cellular aging in the supporting health through nutrition and exercise study. Psychosom. Med. 80(7), 609–619 (2018).
van den Munckhof, I. et al. Sex differences in fat distribution influence the association between BMI and arterial stiffness. J. Hypertens 35(6), 1219–1225 (2017).
Marassi, M. & Fadini, G. P. The cardio-renal-metabolic connection: A review of the evidence. Cardiovasc. Diabetol. 22(1), 195 (2023).
Damman, K. et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 35(7), 455–469 (2014).
Shen, Y. et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 55(1), 66–76 (2017).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865), 813–820 (2001).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107(9), 1058–1070 (2010).
Lim, H. S., MacFadyen, R. J. & Lip, G. Y. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch. Intern. Med. 164(16), 1737–1748 (2004).
Hillege, H. L. et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102(2), 203–210 (2000).
Neyra, J. A., Hu, M. C. & Moe, O. W. Klotho in clinical nephrology: Diagnostic and therapeutic implications. Clin. J. Am. Soc. Nephrol. 16(1), 162–176 (2020).
Drew, D. A. et al. Association between soluble klotho and change in kidney function: The health aging and body composition study. J. Am. Soc. Nephrol. 28(6), 1859–1866 (2017).
Rotondi, S. et al. Soluble α-Klotho serum levels in chronic kidney disease. Int. J. Endocrinol. 2015, 872193 (2015).
Edmonston, D., Grabner, A. & Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 21(1), 11–24 (2024).
Acknowledgements
We sincerely extend our gratitude to all NHANES participants, and all the effort put in by the NHANES team.
Funding
This work was supported in part by the Qilu Health Excellent Young Talents Project (Lu Wei Ren Zi [2020] No. 3); the Shandong Province Chinese Medicine Science and Technology Project (no. M-2022246).
Author information
Authors and Affiliations
Contributions
S.C., Y.K. and L.C. contributed to the study concept and design. S.C., Y.K., N.W., K.N., H.C., Z.Z., contributed to data acquisition. S.C., L.C., drafted the manuscript. All authors contributed to critical revisions of the manuscript for important intellectual content. S.C. was responsible for statistical analysis. N.K., L.L. obtained funding. L.C., L.L. provided study supervision. All participants of NHANES provided written informed consent.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Kong, Y., Wang, N. et al. Association between weight change and serum anti-aging protein α-Klotho: a cross-sectional study in middle-aged and older adults. Sci Rep 14, 18624 (2024). https://doi.org/10.1038/s41598-024-69556-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69556-4
- Springer Nature Limited