Abstract
Fuchs endothelial corneal dystrophy is a heterogenous disease with multifactorial etiology, and genetic, epigenetic, and exogenous factors contributing to its pathogenesis. DNA damage plays a significant role, with ultraviolet-A (UV-A) emerging as a key contributing factor. We investigate the potential application of neuropeptide α-melanocyte stimulating hormone (α-MSH) in mitigating oxidative stress induced endothelial damage. First, we examined the effects of α-MSH on a cultured human corneal endothelial cell line (HCEnC-21T) exposed to hydrogen peroxide (H2O2) induced oxidative DNA damage. We performed immunofluorescence and flow cytometry to assess DNA damage and cell death in the cultured cells. Additionally, we used an established mouse model that utilizes ultraviolet light to induce corneal endothelial cell damage resulting in decreased CEnC number, increased cell size variability, and decreased percentage of hexagonal cells. This endothelial decompensation leads to an increase in corneal thickness. Following UV-A exposure, the mice were systemically treated with α-MSH, either immediately after exposure (early treatment) or beginning two weeks post-exposure (delayed treatment). To evaluate treatment efficacy, we analyzed CEnC density and morphology using in vivo confocal microscopy, and central corneal thickness using anterior segment optical coherence tomography. Our findings demonstrated that α-MSH treatment effectively protects HCEnC-21T from free-radical induced oxidative DNA damage and subsequent cell death. In vivo, α-MSH treatment, mitigated the loss of CEnC density, deterioration of cell morphology and suppression of the resultant corneal swelling. These results underline the potential application of α-MSH as a therapeutic agent for mitigating corneal endothelial damage.
Similar content being viewed by others
Introduction
With an estimated 7.33% global prevalence, Fuchs Endothelial Corneal Dystrophy (FECD) is the most common primary corneal endothelial dystrophy and the leading indication for corneal transplantation in the United States1. FECD is characterized by progressive decline in corneal endothelial cell (CEnC) density, alteration in cell morphology (pleomorphism) and variability in size (polymegethism)2,3. Progressive CEnC damage results in disruption of the endothelial barrier function leading to corneal edema, bullae formation, and subsequent stromal cell death and subepithelial fibrosis. These pathological changes lead to a decrease in corneal transparency and vision loss4,5. The pathogenesis of FECD is complex and can result from a genetic predisposition rendering CEnCs more susceptible to oxidative stress from environmental exposure4,6,7,8. UV-A (320–380 nm wavelength) radiation has been shown to play a critical role in FECD pathogenesis through reactive oxygen species (ROS) generation which cause DNA oxidation and damage9. The current treatment modalities entail temporizing measures aimed at decreasing corneal swelling, such as hyperosmotic saline drops, topical steroids, and bandage contact lenses. Definitive treatment is limited to full or partial thickness corneal transplantation, with 54.3% of domestic endothelial keratoplasty tissues in 2019 being used for patients with FECD10. In recent years, novel regenerative therapies, such as Rho Kinase inhibitors and Fibroblast growth factor derivatives, have been explored for the treatment of CEnC disorders11,12,13.
Given the well characterized cyto-protective capacity of neuropeptide α-melanocyte stimulating hormone (α-MSH) against UV-induced cell death, and its pigmentation-independent antioxidant response, we explore its potential therapeutic role in FECD14. Our previous work has demonstrated abundant expression of melanocortin receptor 1 (MC1R), a high-affinity receptor for α-melanocyte stimulating hormone (α-MSH), in human and murine CEnCs15. Additionally, we have also demonstrated that α-MSH treatment following acute oxidative and cytokine-induced stress in cold-stored human eye bank corneas led to preservation of CEnC density16. Moreover, we have shown that α-MSH/MC1R signaling plays a critical role in maintaining CEnC viability and donor graft clarity following murine corneal transplantation, and has both pro-regenerative and cytoprotective effects in a murine model of thermal injury17,18. Herein, we utilize a recently characterized and validated murine model of UV-A induced FECD to investigate the early and delayed therapeutic application of α-MSH in suppressing the Fuchs phenotype9,19.
Results
α-MSH supplementation protects against oxidative stress-induced DNA damage
To investigate the cytoprotective effects of α-MSH against oxidative damage, cultured human cells (HCEnC-21 T) were challenged with either low dose (150 μM) or high dose (400 μM) H2O2 for 90 min. This was followed by a 6-h recovery incubation in complete media with or without α-MSH supplementation. Immunofluorescent staining for γ-H2AX foci allowed visualization and quantification of double stranded DNA breaks (DSBs) indexed to Hoescht-stained nuclei (Fig. 1). We observed significantly more DNA damage in untreated cells (12 ± 2 γ-H2AX foci/nucleus) subjected to high dose H2O2 compared to α-MSH treated cells (8 ± 2 γ-H2AX foci/nucleus; p < 0.0001). The cytoprotective action of α-MSH was more pronounced under low dose oxidative stress, with a lower mean γ-H2AX foci/nucleus of 3 ± 2 in untreated cells compared to 1 ± 0.2 with α-MSH treatment (p < 0.0001). These data demonstrate the capacity of α-MSH to preserve genomic integrity, revealing its therapeutic potential to counteract oxidative injury.
α-MSH supplementation suppresses oxidative stress induced double strand DNA breaks in a human corneal endothelial cell line. (A) Representative images of phosphorylated Ser-139 residue of the histone variant H2AX stains by immunofluorescence (Scale bar: 10 μM). (B) Significantly lower number of double stranded DNA breaks was noted (indicated by lower number of γ-H2AX foci per cell) in the α-MSH treatment group compared to untreated low and high oxidative stress controls. The data are represented as mean ± SEM. Pairs of groups were compared using student’s t-test. ns: no significant difference, *p < 0.05, **p < 0.01, ***p < 0.001.
α-MSH attenuates oxidative stress-induced CEnC death
HCEnC-21 T were challenged with low dose oxidative stress (150 μM H2O2) for 90 min, followed by 24-h recovery in complete medium (Fig. 2). The in vitro experiment included two α-MSH treatment groups: (1) cells that were treated during peroxide exposure and recovery, or (2) treatment solely during the recovery period. Quantitative analyses of live, apoptotic (caspase-3/7 +), and necrotic (SYTOX + and caspase-3/7 +) cells were performed. Compared to untreated control (H2O2 challenge), peroxide-challenged α-MSH treated cells showed a tenfold decrease in apoptosis (p = 0.0001) and fourfold decrease in necrosis (p < 0.0001). Moreover, we observed comparable reduction in apoptosis and necrosis frequencies in both treatment groups. Despite sufficient recovery time, substantial cytotoxicity persisted in H2O2 challenged, untreated cells. In contrast, α-MSH supplementation during the post-exposure period attenuated overall oxidant-induced cell death (Fig. 2).
α-MSH supplementation confers cyto-protection, decreasing cell death after exposure to oxidative stress. (A) HCEnC-21T underwent 150 μM H2O2 exposure for 90 min with or without α-MSH. Subsequently, the HCEnC-21T were washed and cultured for 24 h in medium supplemented with α-MSH. (B) Representative flow cytometry plots of single cells that were stained using FITC (CellEventTM Caspase-3/7 Green Detection Reagent) and PerCP-Cy5.5 (SYTOXTM AADvancedTM Dead Cell Stain) signals. Gating delineated three distinct cell populations—live cells (L) denoted by dual negative staining, apoptotic cells (A) distinguished by singular FITC positivity, and necrotic cells (N) identified through dual FITC and PerCP-Cy5.5 positive staining. (B) Quantification of cell population frequencies presented as bar graphs. The data are presented as mean ± SEM. Statistical comparisons between groups was conducted using one-way ANOVA with Bonferroni correction, ****p ≤ 0.0001, ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.01.
α-MSH treatment mitigates UV-A-induced loss of corneal endothelial cells in vivo
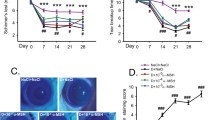
Using a previously established murine model of UV-A induced FECD, we tested the effect of α-MSH treatment on preserving corneal endothelial cell density (Fig. 3B). We injected α-MSH intra-peritoneally, and the ELISA performed 90 min after injection demonstrated a 1.67-fold increase in peptide concentration in the aqueous humor (Supplementary Fig. 1). In untreated FECD controls, a marked 60% decline in mean CEnC densities was observed on day 84 (880 ± 70 cells/mm2) compared to baseline (2215 ± 49 cells/mm2; p < 0.0001). Early α-MSH supplementation (initiated on day 1 post-UV-A exposure) resulted in only 16% cell reduction by day 84 (1850 ± 109 cells/mm2), compared to baseline (2217 ± 36 cells/mm2, p < 0.0001).
α-MSH mitigates UV-A-induced loss of corneal endothelial cells. (A) Representative in vivo confocal microscopy images of corneal endothelial cells prior to UV-A exposure and at post-irradiation days 14, 28, 56, and 84. (B) Quantification of corneal endothelial cell density over time was performed by flex-center method using CellChekD + software. (C) Analysis of endothelial cell polymegathism and hexagonality also performed by CellChekD + software. The data are presented as mean ± SEM, including N = 10 mice per group. The statistical comparisons between groups at each time point was performed by two-way ANOVA with Tukey’s multiple comparisons test. ns: no significant difference, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Delayed α-MSH treatment was initiated 2 weeks after UV-A exposure. We observed a 26% reduction in CEnC densities before initiation of treatment (1702 ± 168 cells/mm2) that was comparable to reduction in CEnC number in the control group (1654 ± 143 cells/mm2, p > 0.99). At day 28, we noted further reduction of CEnC densities in both the delayed treatment (1301 ± 124 cells/mm2) and control (1338 ± 141 cells/mm2) groups. However, on subsequent follow-ups on days 56 (13,912 ± 79 cells/mm2 vs. 976 ± 94 cells/mm2; p = 0.0093) and 84 (1297 ± 96 cells/mm2 vs. 879 ± 70 cells/mm2; p = 0.016), the CEnC densities in the delayed treatment group were maintained, whereas there was progressive loss of CEnC densities in the control group. These data demonstrate the efficacy of α-MSH in maintaining CEnC density in experimental Fuchs dystrophy, despite initiation after significant cell loss. These findings underline the potential therapeutic application of α-MSH in individuals with early FECD.
α-MSH therapy maintains CEnC hexagonal morphology and uniform cell size in experimental Fuchs dystrophy
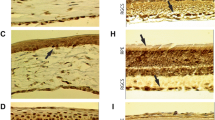
The characteristic hexagonal morphology and homogenous CEnC size are integral to the monolayer’s barrier and pump functions. The UV-A induced FECD mouse model also recapitulates the characteristic morphological changes in CEnCs, clinically observed in Fuchs Dystrophy (Fig. 4). In untreated FECD controls, we observed a marked increase in coefficient of variation (CV; signifying variation in CEnC size) at day 84 (58.7 ± 4.4) compared to baseline (36.3 ± 1.9; p < 0.0001). Early α-MSH treatment resulted preservation of CV on day 84 (31.3 ± 0.8) significantly lower than untreated FECD controls (58.7 ± 4.4; p < 0.0001), and comparable to baseline exposure. We had similar observation on days 28 and 56 post-UV-A exposure in the α-MSH treatment compared to untreated FECD controls. Concurrently, the percentage of cells retaining the normal hexagonal architecture steeply declined from 58.37 ± 2.5% at baseline to 44.8 ± 1.6% at day 84 in untreated FECD controls. On the contrary, treatment with α-MSH resulted in a significantly lower reduction from 61.25 ± 2.13% at baseline to 54.35 ± 1.39% at 28 days and increased 58.13 ± 1.39% by day 84. The percentage hexagonality in the treatment group was significantly higher than untreated FECD controls (p < 0.0001).
Treatment with α-MSH preserves endothelial morphology, halting corneal decompensation. (A) Representative in vivo confocal microscopy images of untreated FECD controls, early treatment and delayed treatment groups at days 0, 14, 28, 56, and 84. (B) CEnC cell size variability and (C) coefficient of variation and percentage of cells retaining hexagonality, computed by CellChekD + software. The data are presented as mean ± SEM, including N = 10 mice per group. The statistical comparisons between groups at each time point was performed by two-way ANOVA with Tukey’s multiple comparisons test. ns: no significant difference, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In the delayed α-MSH, we observed 20% increase in CV at the initiation of treatment on day 14 that was comparable to the control group (46.7 ± 1.8, 50.1 ± 3.2, p > 0.99). At day 28, we observed further reduction in CV in control (47.3 ± 2.4) group; however, it was maintained and moderately lower in the delayed treatment group (61.6 ± 5.3). By the end of the follow-up, we observed that CV was significantly lower in delayed treatment group (44.8 ± 2.3) compared to control (58.7 ± 4.5, p = 0.04). Despite a significant reduction in hexagonality of CEnCs on day 14, we observed a gradual increase in delayed treatment group, and was significantly higher than untreated controls on day 84 (50.0 ± 1.1% vs. 44.8 ± 1.6%, p = 0.04). These data demonstrate the efficacy of α-MSH in preserving the signature morphological features of the corneal endothelium in Fuchs dystrophy.
Preserved CEnC morphology maintains function of the endothelial barrier and integrity of the cornea
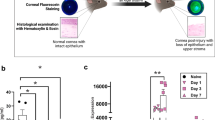
Progressive alterations in cell morphology and density that define Fuchs dystrophy lead to disruption of endothelial barrier and pump functions, resulting in corneal swelling and edema (Fig. 5). In untreated FECD corneas, central corneal thickness increased from 91.14 ± 2.91 μM at baseline to 140 ± 7.79 μM by day 84 post-UV exposure, indicating corneal edema. Early α-MSH intervention maintained CCT comparable to baseline throughout follow-up and measured 90.16 ± 2.4 μM at study completion, significantly lower than the untreated FECD controls (p < 0.0001). At day 14 post-UV exposure, the time-point at which we began delayed α-MSH treatment, CCT had increased to 101.42 ± 6.4 μM. Treatment with α-MSH stabilized the corneal thickness which plateaued throughout follow-up. By day 84, CCT was significantly lower in the delayed treatment group compared to untreated FECD controls (98.4 ± 5.68 μM vs. 140 ± 7.79 μM, p < 0.0001). By preserving endothelial cell density and morphology, α-MSH supplementation prevented the corneal edema associated endothelial barrier dysfunction as observed in Fuchs dystrophy.
Treatment with α-MSH preserves central corneal thickness preventing corneal decompensation. (A) Assessment of mean central corneal thickness (CCT) at baseline and during the follow up period; data are presented as mean ± SEM. Statistical comparisons between groups at each timepoint were conducted by two-way ANOVA with Tukey’s multiple comparisons test. *denotes significant difference between early α-MSH treatment group compared to untreated controls, *p < 0.05, **p < 0.01, ***p < 0.001. # denotes significant difference between delayed α-MSH treatment group compared to untreated controls ##p < 0.01 (B) Representative optical coherence tomography (OCT) images of mouse corneas at day 84 following UV-A irradiation and α-MSH treatment. (C) Representative confocal images of corneal endothelial cell whole mounts at day 84, immunolabeled for zonula occludens-1 (ZO-1, green) and counterstained with DAPI (blue).
Discussion
FECD is a complex, chronic disorder, resulting from the multifactorial interplay of genes and the environment. The recent years have seen a steady shift from penetrating keratoplasty (PK) to the better-tolerated endothelial keratoplasty (EK), for treating FECD20. However, transplantation remains a sub-optimal therapeutic modality. First, EK procedures require substantial resource utilization including graft preparation, surgical suite availability, and transplant re-bubbling in a significant number of cases21. Second, while EK rejection rates remain low, there is a progressive major decline in CEnC number in the years following the surgery22. Third, the vast majority of patients with FECD, including many of whom have symptoms, do not undergo surgery23. Thus, any therapy that can delay or reverse the progressive degeneration of the corneal endothelium can potentially have a major impact for patients who are at risk of CEnC loss.
Significant advances have demonstrated the interplay of genetic, epigenetic, and exogenous factors in an array of ocular pathologies. As in FECD, exposure to UV radiation, exacerbates progression of age-related macular degeneration (AMD), pterygia formation, and keratoconus progression, highlighting the importance of protective measures in managing these diseases24,25,26,27,28,29. Emerging evidence also underlines the parallels between FECD and neurodegenerative diseases30. The therapeutic application of neuropeptides has shown promise for treating diseases such as Alzheimer associated dementia, Parkinson’s disease and multiple sclerosis31. Building on these breakthroughs, studies have demonstrated the potential therapeutic application of neuropeptides such as vasoactive intestinal peptide and α-MSH for their cyto-protective functions to enhance CEnC survival18,32. Herein, we demonstrate the therapeutic efficacy of α-MSH in mitigating oxidative stress induced CEnC damage and preserving endothelial function in a murine model of FECD. Our findings reveal that α-MSH treatment attenuates endothelial cell death, preserves characteristic hexagonal and uniform morphology of CEnCs, and prevents the associated pathological changes induced by oxidative stress.
α-MSH is an evolutionarily conserved, 13 amino acid peptide, derived from the cleavage of proopiomelanocortin (POMC)33. Initially considered solely as a pigment-stimulating hormone, it is now known to have anti-inflammatory, analgesic, and antioxidative functions34,35. The capacity of α-MSH to suppress oxidative damaged has been extensively described in various tissues. For example, following UV-B exposure, keratinocyte treatment with α-MSH activates XPA binding protein 1 (XAB1) leading to nuclear translocation of XPA, a critical factor in nucleotide excision repair signaling, upregulating DNA repair36. Following H2O2-induced oxidative challenge in retinal pigment epithelial cells, α-MSH-MC1R signaling activates Akt/mammalian target of rapamycin (mTOR) and Erk1/2 signaling, increasing cell survival37.
In addition to sharing a neural crest origin, CEnC resemble skin melanocytes in having a limited regenerative capacity, high metabolic activity, and high exposure to UV radiation throughout life38,39,40. As cells originating from the same developmental lineage often retain conserved signaling pathways, we speculate that CEnC exhibit a similar molecular response to α-MSH as melanocytes for DNA repair and cyto-protection. In melanocytes, α-MSH binds to MC1R, which leads to increase in cAMP levels, subsequently activating PKA, that in turn increases expression of transcription factors p53 and Nuclear factor erythroid 2-related factor 2 (Nrf-2)41. This results in increased expression of antioxidant genes, repair of UVR-induced DNA damage, and promotion of cell survival42,43. Kakot et al. further demonstrated that α-MSH supplementation in melanocytes increases Nrf-2 and subsequently Nrf-2 dependent antioxidant gene expression44. Loss of Nrf2 activity has been shown to be a driver of the phenotypic and morphologic changes in FECD, implicating that targeting its pharmacologic precursors in CEnC could be a promising avenue of treatment45.
In cellular models, the concentrations of H2O2 mostly utilized to induce oxidative damage range between 100 and 500 μM to induce oxidative damage46. In our in vitro experiments, we challenge HCEnC-21T with low (150 μM) or high (400 μM) concentrations of H2O2 to simulate the oxidative stress pathways. This approach effectively mimics the downstream effects of various oxidative stressors describe in FECD, leading to the oxidation of DNA bases and subsequent double-stranded DNA breaks (DSBs)10,46. Phosphorylation of Ser-139 residues of the histone variant H2AX results in what is known as γ-H2AX. It is the first step of recruiting and localizing DNA repair genes making quantification of γ-H2AX a direct indicator of DSBs47,48. While UV-A comprises the predominantly transmitted ultraviolet spectrum reaching the endothelium, its deleterious effects on DNA integrity are mediated through the generation of reactive oxygen species, such as H2O2, that subsequently induce DNA damage. Therefore our H2O2 challenge recapitulates the mechanism of UV-A-induced genotoxicity49,50. The significantly lower γ-H2AX staining noted in HCEnC-21T treated with α-MSH treatment implies (1) anti-oxidant function of the neuropeptide preventing DSBs or (2) upregulation in DNA repair pathways leading to faster DSB repair. Our in vitro data reveal that α-MSH acts at the level of preserving genomic integrity. This reduction in DNA damage subsequently attenuated endothelial cell death and cells treated with α-MSH had a significantly lower frequency of apoptotic cells.
α-MSH is constitutively expressed in aqueous humor and vitreous fluid, and is known to play a pivotal role in maintaining ocular immune privilege35,51. Retinal pigment epithelial (RPE) cells have been shown to produce α-MSH through the proteolytic cleavage of POMC by local enzymes52. Iris and ciliary body cells also express these enzymes, implicating multiple intraocular tissues in the production of α-MSH53. In our experiments, we administer α-MSH systemically as topical administration of the peptide would not serve as a reliable modality of therapy due to its limited trans-corneal penetration. In addition, repeated injections of therapeutics into the conjunctiva induces ocular surface inflammation and tissue edema that can impact corneal parameters due to swelling and alterations in the biodynamics of the tear film. Therefore, we adopted a reliable method of administering α-MSH at a non-ocular site, that permits penetration into ocular tissues confirmed by a 1.6-fold increase of the peptide’s concentration in the aqueous humor. The present study does not characterize the pharmacokinetic or pharmacodynamic properties governing bioavailability and clearance α-MSH. Rather, this proof-of-concept study aims to elucidate the downstream functional effects elicited by pharmacological activation of melanocortin receptors on CEnC following α-MSH supplementation.
We utilize a non-genetic standardized UV-A induced mouse model of FECD, developed by Liu et al., that recapitulates the CEnC phenotypic changes noted in FECD: a decrease in cell density, an increase in pleomorphism and in polymegathism9. Following irradiation, mice develop progressive CEnC loss, characteristic morphologic alterations, and corneal edema. The model also demonstrates a prominently worse phenotype in female mice compared to males—in keeping with the solid body of evidence describing female predominance of FECD, as well as a UV-A fluence dependent dystrophy severity1,10,54. For our experiments, we opted for a fluence of 500 mJ/cm2 in female mice. The selected set-up provided sufficient oxidative stress to induce CEnC changes and tissue decompensation, without causing stromal damage and opacification, noted at the higher fluences, that could obscure our in vivo visualization of the endothelium during the 12-week follow up. In UV-induced FECD controls, a significant decrease in CEnC density, and an increase in polymegathism and pleomorphism was observed during the first 4 weeks and continued during the entire follow-up period; a similar trend was observed in loss of cell hexagonality and variability in cell sizes. These changes contribute to the subsequent corneal swelling and edema, indicative of impaired endothelial barrier functions. In contrast, early α-MSH therapy initiated immediately after UV-A exposure drastically suppressed endothelial cell death, maintaining CEnC counts at the end of follow to over 80% of pre-irradiation baselines. The choice to initiate delayed α-MSH treatment at 2 weeks post-UV exposure has a higher translational impact reflecting the later time point at which FECD patients present with phenotypic cellular changes. With delayed treated, a plateau in CEnC decline was observed starting at week 4, along with preservation of CEnC morphology, reflecting improved endothelial health. Importantly, our data show that even though with delayed treatment the level of endothelial cell protection is not as high as that conferred by early treatment, cell numbers remain high enough to maintain normal corneal thickness. In summary, our findings suggest that α-MSH or melanocortin receptor agonism may hold promise in attenuating CEnC damage and preserving function.
Materials and methods
All mice included in the experiments were housed at the Schepens Eye Research Institute’s animal vivarium and were treated in accordance with the Association for Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research. The Schepens Eye Research Institutional Animal Care and Use Committee approved all procedures.
Cell culture
Immortalized human corneal endothelial cells (HCEnC-21T, P30) were obtained from Dr. Ula Jurkunas’s lab (Schepens Eye Research Institute, Boston, MA) and cultured in Chen’s medium. Chen’s media was prepared by combining various components to achieve the desired concentrations. 460 ml of Optimem-I (11058021, Life Technologies, Carlsbad, CA) was used as the basal medium. Then, calcium chloride (C7902, Sigma, St. Louis, MO) was added to achieve a final concentration of 200 mg/l. Next, chondroitin sulfate (C9819, Sigma, St. Louis, MO) was incorporated to reach a concentration of 0.08%. Pituitary Extract (502-102, Gemini Bioproducts, West Sacramento, CA) was added to achieve 66 µg/ml, and EGF (01-101, Millipore, Burlington, MA) was added to reach a final concentration of 5 ng/ml. Finally, FBS (10082147, Life Technologies, Carlsbad, CA) was incorporated for an 8% final concentration, Gentamycin (15750078, Life Technologies, Carlsbad, CA) and Antibiotic/Antimycotic solution (15240062, Life Technologies, Carlsbad, CA) were included to maintain a 1X concentration of each. The media was mixed thoroughly, sterilized by filtration, and stored at 4 °C throughout use. Experiments were performed on cells (passage 31–33) at an 80% confluency.
Oxidative challenge
Cells were cultured in Chen’s medium until 80% confluency and then challenged with hydrogen peroxide (H2O2) (Sigma H1009-5ML) at low (150 μM) or high (400 μM) concentrations for 90 min in the presence or absence of α-MSH. H2O2 was then washed off thrice with PBS, and cells were cultured for either 6 h (γ-H2AX staining for double stranded DNA breaks) or 24 h (CellEvent Caspase-3/7 and SYTOX AADvanced Dead Cell Stain), in the presence or absence of α-MSH.
Immunocytochemistry staining and quantification
Cells cultures were performed in 18 well chamber slides (81,816, Ibidi USA Inc, Fitchburg WI) and fixed, permeabilized with cold methanol (179,957, Sigma-Aldrich, St. Louis, MO, USA) and TritonX-100 (04,802,921, MP Biomedicals, Irvine, CA). Subsequently, the cells were stained with phosphorylated histone 2AX (γ-H2AX) (560,445, BD Biosciences Inc., Franklin Lakes NJ) overnight in 5% BSA-PBS at 4 °C. Cell nuclei were counter stained with Hoechst 33,342. γ-H2AX expression was detected using confocal microscopy (×100 to ×400 magnification; Leica TCS-SP8; Leica, Wetzlar, Germany). Loci quantification was performed on Image J.
Analysis of apoptotic and necrotic cell death
Cells were harvested by gentle scraping, washed with PBS, and stained with CellEvent Caspase-3/7 Green Detection Reagent and SYTOX AADvanced Dead Cell Stain as per the guidelines provided by the manufacturer (C10427, ThermoFisher Scientific, Waltham, MA). Flow cytometry analysis was performed using the LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ). The CellEvent Caspase-3/7 Green Detection Reagent fluorescence was measured using a 530/30 bandpass filter or its equivalent (FITC), while the SYTOX AADvanced Dead Cell Stain fluorescence was detected using a 690/50 bandpass filter (PerCP-Cy5.5).
Experimental animals and disease induction
8-week-old C57BL/6 wild-type female mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were anesthetized with a combined dose of ketamine (100 mg/kg) and xylazine (20 mg/kg), administered intraperitoneally. We utilized a previously established non-genetic animal model that recapitulates the morphological and molecular changes noted in FECD with high fidelity, by exposing murine corneas to UV-A light9. Briefly, a UV-A light source (M365LP1; Thorlabs Inc., Newtown NJ) with an emission peak of 365 nm, bandwidth of 9 nm (FWHM), and irradiance of 398 mW/cm2 was focused to illuminate a central 4-mm of the mouse’s right cornea. Energy was measured with a thermal power sensor head (S425C, Thorlabs Inc.) and energy meter console (PM100D, Thorlabs Inc.) and the UV-A exposure time was adjusted to deliver a total intensity of 500 J/cm2. The mouse’s head, contralateral eye, and body were covered with UV opaque heat retention drapes (Locus Technologies Inc., Manchester MD). Each mouse received a single exposure of UV-A at the start of the experiment. At the end of the follow-up, euthanasia was performed by CO2 inhalation overdose. This study is reported in accordance with ARRIVE guidelines.
α-MSH treatment
Lyophilized α-MSH powder (Sigma-Aldrich Inc., Waltham MA) was reconstituted in sterile phosphate-buffered saline (PBS) following the manufacturer’s guidelines to prepare a 10−4 M α-MSH stock solution. In the early treatment group, mice received a 0.1 ml intraperitoneal injection of the α-MSH solution immediately after UV-A exposure, delivering a dose of 1.67 µg/g which was given thrice weekly for the duration of treatment. In the delayed treatment group, α-MSH treatment began at 2 weeks after UV-A exposure and was continued thrice weekly for the remaining 10 weeks (Fig. 3A). Increased bioavailability of α-MSH in the aqueous humor following systemic administration was confirmed with ELISA (Supplementary Fig. 1).
Detection of α-MSH in aqueous humor
90 min following intraperitoneal injection of α-MSH, aqueous humor was collected and immediately placed on ice. ELISA was performed to detect alpha-MSH concentration using the Mouse Alpha-Melanocyte Stimulating Hormone ELISA Kit (Cat. No. MBS054363, MyBioSource, San Diego, CA, USA).
In vivo imaging
Prior to irradiation, and at weeks 2, 4, 8, and 12 post UV-A exposure, corneal slit lamp bio-microscopy images were captured with the mounted camera (Nikon D100, Tokyo). Epithelial integrity was assessed by instilling fluorescein (1 μL of 2.5% in PBS; Sigma-Aldrich Inc.) in the conjunctival sac and punctate staining was observed under cobalt blue light.
Optical coherence tomography (OCT) was used to capture images of the anterior segment (Bioptigen Spectral Domain Ophthalmic Imaging System Envisu R2200 with 12 mm telecentric lens to scan the cornea; Bioptigen Inc., Durham NC). Subsequently, central corneal thickness (CCT) was calculated using built-in software. For CEnC imaging, we utilized laser scanning in vivo confocal microscopy using the Heidelberg Retina Tomograph III (HRT III) with Rostock Corneal Module (RCM) (Heidelberg Engineering GmbH, Heidelberg, Germany). Coronal 2D sections of the corneal endothelium (400 × 400 mm) were acquired.
Analysis of CE cell density
The acquired HRT images were analyzed using the KONAN CELLChek software (Konan Medical USA Inc., Irvine, CA). Three images per mouse per time point, with at least 50 visible cells, were selected (Fig. 3B). As recommended by the software manufacturer, a blinded observer selected the center of each cell to be analyzed, and the semi-automated flex-center method was utilized. The mean cell density, cell hexagonality, and cell size variability was calculated for each mouse (Fig. 3C).
Whole mount cornea staining
16% paraformaldehyde (PFA) (28,906, Thermofisher Scientific, Waltham, MA) was freshly diluted in PBS to obtain a 4% PFA solution. Mouse eyeballs were fixed in 4% PFA for 30 min at room temperature. The corneas were then gently dissected and permeabilized with 0.2% Triton X-100 in PBS for 30 min and blocked in 3% bovine serum albumin (BSA)-PBS for 1 h. The cornea cup was incubated with anti-ZO-1 antibody (339,188; ThermoFisher Scientific, Waltham, MA) in 3% BSA-PBS at 4 °C for 48 h. The corneal cup was flattened by 4–6 radial cuts and mounted using DAPI mounting medium for cell indexing (H-1200; Vector Labs, Newark CA). ZO-1 expression was detected using confocal microscopy (× 100 to × 400 magnification; Leica TCS-SP8; Leica, Wetzlar, Germany).
Statistical analysis
For comparing quantitative data between two groups, two-tailed t-tests were performed. One-way analysis of variance (ANOVA) with Bonferroni correction was used to compare the differences in the means of three or more independent groups. The results are presented as mean ± SEM. The differences were considered statistically significant was considered when p < 0.05.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Aiello, F., Gallo Afflitto, G., Ceccarelli, F., Cesareo, M. & Nucci, C. Global prevalence of Fuchs endothelial corneal dystrophy (FECD) in adult population: A systematic review and meta-analysis. J. Ophthalmol. 2022, 3091695 (2022).
Vedana, G., Villarreal, G. & Jun, A. S. Fuchs endothelial corneal dystrophy: Current perspectives. Clin. Ophthalmol. Auckl. NZ 10, 321–330 (2016).
Jun, A. S. One hundred years of Fuchs’ dystrophy. Ophthalmology 117, 859–860 (2010).
Hamill, C. E., Schmedt, T. & Jurkunas, U. Fuchs endothelial cornea dystrophy: A review of the genetics behind disease development. Semin. Ophthalmol. 28, 281–286 (2013).
Bourne, W. M. Biology of the corneal endothelium in health and disease. Eye Lond. Engl. 17, 912–918 (2003).
Jurkunas, U. V., Bitar, M. S., Funaki, T. & Azizi, B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am. J. Pathol. 177, 2278–2289 (2010).
Patel, S. P. et al. Effect of physiological oxygen on primary human corneal endothelial cell cultures. Transl. Vis. Sci. Technol. 11, 33 (2022).
Jurkunas, U. V. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea 37(Suppl 1), S50–S54 (2018).
Liu, C. et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. U. S. A. 117, 573–583 (2020).
Ong Tone, S. et al. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 80, 100863 (2021).
Eveleth, D., Pizzuto, S., Weant, J., Jenkins-Eveleth, J. & Bradshaw, R. A. Proliferation of human corneal endothelia in organ culture stimulated by wounding and the engineered human fibroblast growth factor 1 derivative TTHX1114. J. Ocul. Pharmacol. Ther. 36, 686–696 (2020).
Meekins, L. C. et al. Corneal endothelial cell migration and proliferation enhanced by rho kinase (ROCK) inhibitors in in vitro and in vivo models. Invest. Ophthalmol. Vis. Sci. 57, 6731–6738 (2016).
Okumura, N. et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 6, 26113 (2016).
Guida, S., Guida, G. & Goding, C. R. MC1R functions, expression, and implications for targeted therapy. J. Invest. Dermatol. 142, 293–302 (2022).
Lužnik, Z. et al. Association of α-Melanocyte–stimulating hormone with corneal endothelial cell survival during oxidative stress and inflammation-induced cell loss in donor tissue. JAMA Ophthalmol. 138, 1–5 (2020).
Marzidovšek, Z. L. et al. The neuropeptide alpha-melanocyte–stimulating hormone is critical for corneal endothelial cell protection and graft survival after transplantation. Am. J. Pathol. 192, 270–280 (2022).
Alemi, H. et al. The neuropeptide α-Melanocyte–stimulating hormone prevents persistent corneal edema following injury. Am. J. Pathol. 194(1), 150–164 (2023).
Wang, S. et al. Therapeutic effects of stimulating the melanocortin pathway in regulating ocular inflammation and cell death. Biomolecules 14, 169 (2024).
Wang, Q. et al. Heterogeneity of human corneal endothelium implicates lncRNA NEAT1 in Fuchs endothelial corneal dystrophy. Mol. Ther. Nucleic Acids 27, 880–893 (2022).
Statistical Report. EBAA. https://restoresight.org/members/publications/statistical-report/.
Roberts, H. W. & de Benito-Llopis, L. Comparison of repeat penetrating keratoplasty, DSAEK and DMEK for the management of endothelial failure of previous PK. Eye https://doi.org/10.1038/s41433-023-02561-5 (2023).
Nanavaty, M. A., Wang, X. & Shortt, A. J. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst. Rev. 2, CD008420 (2014).
Heckenlaible, N. J. et al. Predictors of receiving keratoplasty for Fuchs’ endothelial corneal dystrophy among medicare beneficiaries. Ophthalmology 130, 28–38 (2023).
Chalam, K. V., Khetpal, V., Rusovici, R. & Balaiya, S. A review: Role of ultraviolet radiation in age-related macular degeneration. Eye Contact Lens 37, 225–232 (2011).
Zhou, W. P., Zhu, Y. F., Zhang, B., Qiu, W. Y. & Yao, Y. F. The role of ultraviolet radiation in the pathogenesis of pterygia (review). Mol. Med. Rep. 14, 3–15 (2016).
Maurizi, E. et al. A novel role for CRIM1 in the corneal response to UV and pterygium development. Exp. Eye Res. 179, 75–92 (2019).
Liu, T., Liu, Y., Xie, L., He, X. & Bai, J. Progress in the pathogenesis of pterygium. Curr. Eye Res. 38, 1191–1197 (2013).
Roy, S. et al. Interplay between hereditary and environmental factors to establish an in vitro disease model of keratoconus. Drug Discov. Today 24, 403–416 (2019).
Lucas, S. E. M. & Burdon, K. P. Genetic and environmental risk factors for keratoconus. Annu. Rev. Vis. Sci. 6, 25–46 (2020).
Li, C. et al. Roles of neuropeptide Y in neurodegenerative and neuroimmune diseases. Front. Neurosci. 13, 869 (2019).
Satitpitakul, V. et al. Vasoactive intestinal peptide promotes corneal allograft survival. Am. J. Pathol. 188, 2016–2024 (2018).
Kheirkhah, A., Satitpitakul, V., Hamrah, P. & Dana, R. Patients with dry eye disease and low subbasal nerve density are at high risk for an accelerated corneal endothelial cell loss. Cornea 36, 196–201 (2017).
Singh, M. & Mukhopadhyay, K. Alpha-melanocyte stimulating hormone: An emerging anti-inflammatory antimicrobial peptide. BioMed Res. Int. 2014, 874610 (2014).
D’Agostino, G. & Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. Berl. Ger. 88, 1195–1201 (2010).
Wu, C. L. et al. The multifunctional human ocular melanocortin system. Prog. Retin. Eye Res. 95, 101187 (2023).
Dong, L. et al. Melanocyte-stimulating hormone directly enhances UV-induced DNA repair in keratinocytes by an XPA-dependent mechanism. Cancer Res. 70, 3547–3556 (2010).
Cheng, L. et al. Alpha-melanocyte stimulating hormone protects retinal pigment epithelium cells from oxidative stress through activation of melanocortin 1 receptor-Akt-mTOR signaling. Biochem. Biophys. Res. Commun. 443, 447–452 (2014).
Williams, A. L. & Bohnsack, B. L. The ocular neural crest: Specification, migration, and then what?. Front. Cell Dev. Biol. 8, 595896 (2020).
Cichorek, M., Wachulska, M., Stasiewicz, A. & Tymińska, A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol. Dermatol. Alergol. 30, 30–41 (2013).
Hatou, S. & Shimmura, S. Review: Corneal endothelial cell derivation methods from ES/iPS cells. Inflamm. Regen. 39, 19 (2019).
Wolf Horrell, E. M., Boulanger, M. C. & D’Orazio, J. A. Melanocortin 1 receptor: structure, function, and regulation. Front. Genet. 7, 95 (2016).
Swope, V. & Abdel-Malek, Z. Significance of the melanocortin 1 and endothelin B receptors in melanocyte homeostasis and prevention of sun-induced genotoxicity. Front. Genet. 7, 146 (2016).
Swope, V. et al. Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell Melanoma Res. 27, 601–610 (2014).
Kokot, A. et al. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology 150, 3197–3206 (2009).
Lovatt, M., Kocaba, V., Hui Neo, D. J., Soh, Y. Q. & Mehta, J. S. Nrf2: A unifying transcription factor in the pathogenesis of Fuchs’ endothelial corneal dystrophy. Redox Biol. 37, 101763 (2020).
Ransy, C., Vaz, C., Lombès, A. & Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 21, 9149 (2020).
Mah, L. J., El-Osta, A. & Karagiannis, T. C. gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 24, 679–686 (2010).
Cleaver, J. E. γH2Ax: Biomarker of damage or functional participant in DNA repair “all that glitters is not gold!”. Photochem. Photobiol. 87, 1230–1239 (2011).
Delic, N. C., Lyons, J. G., Di Girolamo, N. & Halliday, G. M. Damaging effects of ultraviolet radiation on the cornea. Photochem. Photobiol. 93, 920–929 (2017).
Cadet, J., Gentner, N. E., Rozga, B. & Paterson, M. C. Rapid quantitation of ultraviolet-induced thymine-containing dimers in human cell DNA by reversed-phase high-performance liquid chromatography. J. Chromatogr. 280, 99–108 (1983).
Taylor, A. W., Yee, D. G., Nishida, T. & Namba, K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH). Ann. N. Y. Acad. Sci. 917, 239–247 (2000).
Zmijewski, M. A., Sharma, R. K. & Slominski, A. T. Expression of molecular equivalent of hypothalamic–pituitary–adrenal axis in adult retinal pigment epithelium. J. Endocrinol. https://doi.org/10.1677/joe.1.06927 (2007).
Kawanaka, N. & Taylor, A. W. Localized retinal neuropeptide regulation of macrophage and microglial cell functionality. J. Neuroimmunol. 232, 17–25 (2011).
Singh, R. B., Parmar, U. P. S., Kahale, F., Jeng, B. H. & Jhanji, V. Prevalence and economic burden of fuchs endothelial corneal dystrophy in the medicare population in the United States. Cornea https://doi.org/10.1097/ICO.0000000000003416 (2023).
Funding
This study was supported by the National Eye Institute/National Institutes of Health (R21EY032695), awarded to R.D, JY and UJ, the Lions Eye Massachusetts Research Fund to RD and NIH/NEI Core grant (5P30EY003790) awarded to the Schepens Eye Research Institute. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; R.D., F.K., H.A., A.N., U.V.J. and, J.Y. conceived and designed the study; F.K., A.N., N.D., S.W., S.L., acquired, analyzed, or interpreted data; F.K., R.S., T.D., and R.D. drafted the manuscript; T.D., U.V.J., J.Y., and R.D. critically revised the manuscript; F.K., S.L., and A.N. performed statistical analysis; J.Y., U.V.J. and R.D. obtained funding; F.K., T.B., and H.A. provided administrative, technical, or material support; T.D., U.V.J., J.Y., and R.D. supervised the study. R.D. and J.Y. hold equity in SightStream Biotherapeutics.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kahale, F., Alemi, H., Naderi, A. et al. Neuropeptide alpha-Melanocyte stimulating hormone preserves corneal endothelial morphology in a murine model of Fuchs dystrophy. Sci Rep 14, 18842 (2024). https://doi.org/10.1038/s41598-024-69416-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69416-1
- Springer Nature Limited