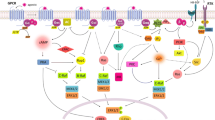
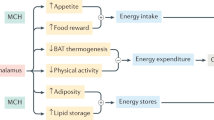
Abstract
Proopiomelanocortin (POMC) is a polypeptide hormone precursor that is expressed in the brain and in peripheral tissues such as in the pituitary gland, immune system, and skin. In the brain, POMC is processed to form several peptides including alpha-melanocyte stimulating hormone (α-MSH). alpha-MSH is expressed in the hypothalamic arcuate nucleus and in the nucleus tractus solitarius of the brainstem where it has a crucial role in the regulation of metabolic functions. Specifically, α-MSH is an anorexigenic peptide. Its production and maturation processes have been shown to be regulated according to the metabolic condition of the organism. This review summarizes our current knowledge on α-MSH processing including its maturation and degradation processes and pharmacological aspects of its manipulation.


Similar content being viewed by others
References
Saper CB, Chou TC, Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295
Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK (2000) Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 423:261–281
Cowley MA, Smart JL, Rubinstein M, Cerdar MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484
Horvath TL, Naftolin F, Kalra SP, Leranth C (1992) Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131:2461–2467
Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS (1997) Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138
Csiffáry A, Görcs TJ, Palkovits M (1990) Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res 506:215–222
Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125
Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW (2001) Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413:794–795
Kitamura T, Feng Y, Kitamura YI, Chua SC Jr, Xu AW, Barsh GS, Rossetti L, Accili D (2006) Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12:534–540
Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klöckener T, Alessi D, Kloppenburg P, Brüning JC (2008) PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 7:291–301
Shyng SL, Nichols CG (1998) Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282:1138–1141
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB (2007) Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228–232
Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA (2009) Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE 4:e8322
Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R (2008) Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28:9989–9996
Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM (1996) Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97
Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK (2008) Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118:1796–1805
Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R et al (2006) Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116:1886–1901
Plum L, Belgardt BF, Bruning JC (2006) Central insulin action in energy and glucose homeostasis. J Clin Invest 116:1761–1766
Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK (1999) Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23:775–786
Morris DL, Rui L (2009) Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 297:E1247–E1259
Steiner DF (1998) The proprotein convertases. Curr Opin Chem Biol 2:31–39
Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG (1991) PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA 88:3564–3568
Abbott CR, Rossi M, Kim MS, AlAhmed SH, Ghatei TGM, Smith DM MA, Bloom SR (2000) Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res 869:203–210
Guo L, Münzberg H, Stuart RC, Nillni EA, Bjørbaek C (2004) N-Acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci USA 101:11797–11802
Perello M, Stuart RC, Nillni EA (2007) Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab 292:E1348–E1357
Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A (2003) Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology 144:760–766
Wilkinson CW (2006) Roles of acetylation and other post-translational modifications in melanocortin function and interactions with endorphins. Peptides 27:453–471
Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH (1995) Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10:135–142
Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S (1997) Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16:303–306
Coll AP, Farooqi IS, Challis BG, Yeo GS, O'Rahilly S (2004) Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab 89:2557–2562
Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD (1997) Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine function by melanocortin peptides. Cell 91:789–798
Clark AJ, McLoughlin L, Grossman A (1993) Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341:461–462
Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD (1993) Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72:827–834
Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW (1997) Melanocortin receptors in leptin effects. Nature 390:349–363
Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD et al (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141
Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD (2000) A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141:3518–3521
Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L et al (2000) Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26:97–102
Jegou S, Boutelet I, Vaudry H (2000) Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol 12:501–505
Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK (2003) Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154
Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992) The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251
Vaisse C, Clement K, Guy-Grand B, Froguel P (1998) A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20:113–114
Yeo GS, Farooqi IS, Challis BG, Jackson RS, O’Rahilly S (2000) The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. Q J Med 93:7–14
Chiesi M, Huppertz C, Hofbauer KG (2001) Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol Sci 22:247–254
Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S (2009) Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest 119:2291–2303
Diament AL, Warden CH (2004) Multiple linked mouse chromosome 7 loci influence body fat mass. Int J Obes Relat Metab Disord 28:199–210
McCarthy JJ, Meyer J, Moliterno DJ, Newby LK, Rogers WJ, Topol EJ (2003) GenQuest multicenter study. Evidence for substantial effect modification by gender in a large-scale genetic association study of the metabolic syndrome among coronary heart disease patients. Hum Genet 114:87–98
Yang HYT, Erdos EG, Chiang TS (1968) New enzymatic route for the inactivation of angiotensin. Nature 218:1224–1226
Yang HYT, Erdos EG, Chiang TS, Jenssen TA, Rodgers JG (1970) Characteristics of an enzyme that inactivates angiotensin II (angiotensinase C). Biochem Pharmacol 19:1201–1211
Tan F, Morris PW, Skidgel RA, Erdos EG (1993) Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). Similarity to both serine carboxypeptidase and prolylendopeptidase families. J Biol Chem 268:16631–16638
Kumamoto K, Stewart TA, Johnson AR, Erdos EG (1981) Prolylcarboxypeptidase (angiotensinase C) in human cultured cells. J Clin Invest 67:210–215
Skidgel RA, Erdos EG (1998) Cellular carboxypeptidases. Immunol Rev 161:129–141
Shariat-Madar Z, Rahimy E, Mahdi F, Schmaier AH (2005) Overexpression of prolylcarboxypeptidase enhances plasma prekallikrein activation on Chinese hamster ovary cells. Am J Physiol Heart Circ Physiol 289(6):H2697–H2703
Smart JL, Tolle V, Low MJ (2006) Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest 116:495–505
Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A (1998) Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157
Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M (2006) The MC4 receptor and control of appetite. Br J Pharmacol 149:815–827
Adan RA, Vanderschuren LJ, la Fleur SE (2008) Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci 29:208–217
Banno R, Arima H, Sato I, Hayashi M, Goto M, Sugimura Y, Murase T, Oiso Y (2004) The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats. Peptides 25:1279–1286
Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP (2005) Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia 48:1621–1626
Tian X, Field T, Mazur AW, Ebetino FH, Wos JA, Crossdoersen D, Pinney BB, Sheldon RJ (2005) Design, synthesis, and evaluation of proline based melanocortin receptor ligands. Bioorg Med Chem Lett 15:2819–2823
Bakshi RK, Hong Q, Tang R, Kalyani RN, Macneil T, Weinberg DH, Van der Ploeg LH, Patchett AA, Nargund RP (2006) Optimization of a privileged structure leading to potent and selective human melanocortin subtype-4 receptor ligands. Bioorg Med Chem Lett 16:1130–1133
Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG (2006) Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther 317:771–777
Holder JR, Haskell-Luevano C (2004) Melanocortin ligands: 30 years of structure–activity relationship (SAR) studies. Med Res Rev 24:325–356
Todorovic A, Haskell-Luevano C (2005) A review of melanocortin receptor small molecule ligands. Peptides 26:2026–2036
Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA et al (2002) A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci USA 99:11381–11386
Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB (2004) Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res 16:51–59
Wessells H, Blevins JE, Vanderah TW (2005) Melanocortinergic control of penile erection. Peptides 26:1972–1977
Versteeg DH, Van Bergen P, Adan RA, De Wildt DJ (1998) Melanocortins and cardiovascular regulation. Eur J Pharmacol 360:1–14
Catania A, Gatti S, Colombo G, Lipton JM (2004) Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29
Vrinten DH, Kalkman CJ, Adan RA, Gispen WH (2001) Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol 429:61–69
Acknowledgments
This work was supported by NIDDK/NIH (Grant no. R01 DK084065).
Disclosure statement
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Agostino, G., Diano, S. alpha-Melanocyte stimulating hormone: production and degradation. J Mol Med 88, 1195–1201 (2010). https://doi.org/10.1007/s00109-010-0651-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0651-0