Abstract
High volume hemofiltration (HVHF) could remove from plasma inflammatory mediators involved in sepsis-associated acute kidney injury (SA-AKI). The IVOIRE trial did not show improvements of outcome and organ dysfunction using HVHF. The aim of this study was to evaluate in vitro the biological effects of plasma of patients treated by HVHF or standard volume hemofiltration (SVHF). We evaluated leukocyte adhesion, apoptosis and functional alterations of endothelial cells (EC) and tubular epithelial cells (TEC). In vitro data were correlated with plasma levels of TNF-α, Fas-Ligand (FasL), CD40-Ligand (CD40L), von Willebrand Factor (vWF) and endothelial-derived microparticles. An experimental model of in vitro hemofiltration using LPS-activated blood was established to assess cytokine mass adsorption during HVHF or SVHF. Plasma concentrations of TNF-ɑ, FasL, CD40L and von Willebrand Factor (vWF) were elevated at the start (d1h0) of both HVHF and SVHF, significantly decreased after 6 h (d1h6), remained stable after 12 h (d1h12) and then newly increased at 48 h (d3h0). Plasma levels of all these molecules were similar between HVHF- and SVHF-treated patients at all time points considered. In addition, the levels of endothelial microparticles remained always elevated, suggesting the presence of a persistent microvascular injury. Plasma from septic patients induced leukocyte adhesion on EC and TEC through up-regulation of adhesion receptors. Moreover, on EC, septic plasma induced a cytotoxic and anti-angiogenic effect. On TEC, septic plasma exerted a direct pro-apoptotic effect via Fas up-regulation and caspase activation, loss of polarity, altered expression of megalin and tight junction molecules with an impaired ability to internalize albumin. The inhibition of plasma-induced cell injury was concomitant to the decrease of TNF-α, Fas-Ligand and CD40-Ligand levels. The protective effect of both HVHF and SVHF was time-limited, since a further increase of circulating mediators and plasma-induced cell injury was observed after 48 h (d3h0). No significant difference of EC/TEC damage were observed using HVHF- or SVHF-treated plasma. The in vitro hemofiltration model confirmed the absence of a significant modulation of cytokine adsorption between HVHF and SVHF. In comparison to SVHF, HVHF did not increase inflammatory cytokine clearance and did not reverse the detrimental effects of septic plasma-induced EC and TEC injury. Further studies using adsorptive membranes are needed to evaluate the potential role of high dose convective therapies in the limitation of the harmful activity of plasma soluble factors involved in SA-AKI.
Trial registration IVOIRE randomized clinical trial; ClinicalTrials.gov (NCT00241228) (18/10/2005).
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is a common syndrome in patients admitted to hospitals and particularly to intensive care unit (ICU)1. AKI is associated with elevated mortality rates2, frequently in a clinical scenario characterized by multiple organ failures due to the development of severe sepsis/septic shock3. In the last years, the pathogenic mechanisms of sepsis-associated AKI (SA-AKI) have been better clarified, showing that they are related not only to tissue hypoperfusion but also to a direct detrimental activity of circulating inflammatory mediators on kidney resident cells3. Our group recently demonstrated that plasma of septic patients induces functional alterations of kidney tubular and glomerular epithelial cells: these modifications observed at cellular and molecular level finally lead to the triggering of apoptosis, the energy-dependent process whereby cells carry out programmed death4. Moreover, apoptosis and functional cell alterations including mitochondrial damage and loss of polarity have been shown to represent key pathogenic features of SA-AKI with a consequent progression toward chronic kidney disease5.
Different extracorporeal blood purification therapies (EBPT) have been shown to remove inflammatory and pro-apoptotic mediators from plasma of patients with sepsis6. Indeed, convection-based strategies unselectively remove several cytokines and chemokines and could consequentially down-regulate the inflammatory response7. On this basis, several studies were directed to evaluate the effect of increasing convective doses on sepsis-associated mortality with contradictory results. Recent studies including the IVOIRE randomized clinical trial failed to demonstrate that High Volume HemoFiltration (HVHF, 70 ml/Kg/hr) lead to a beneficial effect on mortality reduction in refractory septic shock8. However, some beneficial results of HVHF on hemodynamics and limitation of organ dysfunction have been reported8.
The aim of this translational study derived from the IVOIRE clinical trial was to compare the efficiency of HVHF versus Standard Volume HemoFiltration (SVHF) in removing from plasma soluble inflammatory and pro-apoptotic mediators involved in kidney endothelial and tubular epithelial cell injury, typical features of SA-AKI.
Results
Plasma levels of cytokines and endothelial microparticles during HVHF or SVHF
Clinical parameters of 5 patients of the HVHF and 5 of the SVHF group enrolled in the study are reported in Table 1. Plasma concentrations of TNF-ɑ, Fas-Ligand (FasL), CD40-Ligand (CD40L) and von Willebrand Factor (vWF) were elevated at the start (d1h0) of both HVHF and SVHF, significantly decreased after 6 h (d1h6), remained stable after 12 h (d1h12) and then newly increased at 48 h (d3h0). Plasma levels of all these molecules were similar between HVHF- and SVHF-treated patients at all time points considered (Fig. 1). To further assess the presence of microvascular endothelial injury, we also evaluated the percentage of circulating CD31 + endothelial-derived microparticles without finding any significant difference between HVHF and SVHF at all time points considered (percentage of circulating CD31 + endothelial-derived microparticles in HVHF d1h0: 27.2 ± 4.3; HVHF d1h6: 24.8 ± 3.7; HVHF d1h12: 28.2 ± 6.1; HVHF d3h0: 28.8 ± 5.8. SVHF d1h0: 26.4 ± 3.6; SVHF d1h6: 25.3 ± 2.8; SVHF d1h12: 25.6 ± 4,1; SVHF d3h0: 27.4 ± 4.8).
ELISA for TNF‐α, Fas-Ligand (FasL), CD40-Ligand (CD40L) and vWF plasma levels during HVHF or SVHF. TNF‐α, FasL, CD40L and vWF plasma levels at d1h0 were significantly higher than at d1h6 and d1h12 (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, at d3h0 a new significantly increase of all cytokine levels was observed (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were found for SVHF. All experiments were performed in triplicate.
Biological effects of HVHF versus SVHF on septic plasma-induced human kidney endothelial cell (EC) injury
In comparison to plasma drawn from healthy volunteers used as negative experimental control, plasma from septic patients collected at the start of HVHF or SVHF (d1h0) induced a significant increase of both PMN and PBMC adhesion to EC (Fig. 2A,B). Cell adhesion was significantly reduced incubating EC with plasma obtained after 6 h from the beginning of HVHF or SVHF (d1h6) (Fig. 2A,B). No significant differences were found comparing the biological effects of plasma obtained at d1h6 with those collected at d1h12, whereas plasma drawn at d3h0 showed a new significant increase of adhesion of both PMN and PBMC. Of note, HVHF and SVHF plasma showed a similar time-related modulation of leukocyte adhesion. We then evaluated whether this enhanced leukocyte adhesion might be ascribed to the up-regulation of surface adhesion molecules on EC. In respect to control, d1h0 plasma from HVHF patients induced the up-regulation of ICAM-1, VCAM-1 and E-selectin (Fig. 2C). A significant decrease of all surface molecules was detected in presence of both d1h6 and d1h12 plasma, whereas d3h0 plasma induced a new significant increase of adhesion molecule expression (Fig. 2C). Similar results were obtained incubating EC with SVHF plasma (not shown).
Adhesion of granulocytes or monocytes to endothelial cells incubated with plasma collected at different HVHF/SVHF time points. (A, B) Adhesion of granulocytes (PMN in A) or monocytes (PBMC in B) on Endothelial Cells (EC) incubated with plasma collected at different time points of HVHF (black columns) or SVHF (white columns). Incubation of EC with plasma at HVHF/SVHF start (d1h0) increased both PMN and PBMC adhesion (*p < 0.05 d1h0 vs. Vehicle). Plasma samples collected at d1h6 and d1h12 of HVHF/SVHF significantly decreased the number of adherent PMN/PBMC to EC monolayers (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, EC monolayers incubated with d3h0 plasma of HVHF/SVHF showed an enhanced adhesion of PMN/PBMC (#p < 0.05 d3h0 vs. d1h6 or d1h12). (C) FACS analysis of ICAM-1, VCAM-1 and E-selectin expression on EC incubated with plasma collected at different time points of HVHF. In comparison to Vehicle alone, d1h0 plasma up-regulated ICAM-1, VCAM-1 and E-selectin expression on EC surface (*p < 0.05 d1h0 vs. Vehicle). Incubation with plasma collected at d1h6 and d1h12 significantly decreased all adhesion molecule expression (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, d3h0 plasma newly enhanced ICAM-1, VCAM.1 and E-selectin expression (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed using HVHF or SVHF plasma.
In comparison to healthy control, d1h0 septic plasma collected before HVHF or SVHF induced a significant reduction of EC angiogenesis evaluated as the ability to form capillary-like structures on Matrigel-coated plates (Fig. 3A,B). In respect to d1h0 plasma, d1h6 plasma significantly increased EC angiogenesis, whereas no significant differences were observed comparing d1h6 with d1h12 plasma (Fig. 3A,B): by contrast, d3h0 plasma induced a new significant decrease of angiogenesis in respect to both d1h6 and d1h12 time points (Fig. 3A,B). Similar results were observed when the pro-apoptotic potential of septic plasma drawn at different time points after HVHF or SVHF was evaluated by TUNEL assay (Fig. 3C). The modulation of the anti-angiogenic and pro-apoptotic effects of septic plasma was similar between the HVHF and SVHF group.
EC angiogenesis and apoptosis after incubation with plasma collected at different HVHF/SVHF time points. (A, B) Representative images of EC cultured on Matrigel-coated surface (A) and relative quantification of capillary-like structures/field (B) in the presence of plasma collected at different time points after HVHF (black columns) or SVHF (white columns) start. Incubation of EC with d1h0 plasma significantly decreased angiogenesis (*p < 0.05 d1h0 vs. Vehicle). In respect to d1h0 plasma, incubation of EC with d1h6 or d1h12 plasma showed an increased formation of capillary-like structures (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, d3h0 plasma newly decreased EC angiogenic properties (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed using HVHF or SVHF plasma. The number of capillary-like structures on Matrigel was counted in 10 different consecutive images: data are shown as average ± 1SD of 3 different experiments. (C) EC apoptosis in the presence of plasma collected at different time points after HVHF (black columns) or SVHF (white columns) start. Incubation of EC with d1h0 plasma significantly increased apoptosis detected by TUNEL assay (*p < 0.05 d1h0 vs. Vehicle). In respect to d1h0 plasma, incubation of EC with d1h6 or d1h12 plasma showed a decreased rate of apoptotic cells (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, d3h0 plasma newly increased EC apoptosis (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed using HVHF or SVHF plasma. The number of apoptotic cells was counted in 30 different microscopic fields: data are shown as average ± 1SD of 3 different experiments.
Biological effects of HVHF versus SVHF on septic plasma-induced human kidney tubular epithelial cell (TEC) injury
As observed for EC, in comparison to plasma drawn from healthy volunteers, plasma from septic patients collected at the start of HVHF or SVHF (d1h0) induced a significant increase of PMN and PBMC adhesion also on TEC (Fig. 4A,B). Cell adhesion was significantly reduced incubating TEC with plasma obtained after 6 h from the beginning of HVHF or SVHF (d1h6). No significant differences were found comparing the biological effects of plasma obtained at d1h6 with those collected at d1h12, whereas plasma drawn at d3h0 showed a new significant increase of leukocyte adhesion (Fig. 4A,B). HVHF and SVHF plasma showed a similar time-related modulation of PMN and PBMC adhesion (Fig. 4A,B). We then evaluated whether this enhanced leukocyte adhesion could be ascribed to the up-regulation of ICAM-1 and CD40, two surface molecules involved in inflammatory-mediated TEC damage during sepsis. In respect to control plasma, d1h0 plasma from HVHF patients induced the up-regulation of ICAM-1 and CD40. A significant decrease of these surface molecules was detected in presence of both d1h6 and d1h12 plasma, whereas d3h0 plasma induced a new significant increase (Fig. 4C). Similar results were observed incubating TEC with SVHF plasma (not shown).
Adhesion of granulocytes or monocytes to Tubular Cells incubated with plasma collected at different HVHF/SVHF time points. (A, B) Adhesion of granulocytes (PMN in A) or monocytes (PBMC in B) on tubular epithelial cells (TEC) incubated with plasma collected at different time points of HVHF (black columns) or SVHF (white columns). Incubation of TEC with plasma at HVHF/SVHF start (d1h0) increased PMN/PBMC adhesion (*p < 0.05 d1h0 vs. Vehicle). Plasma samples collected at d1h6 and d1h12 of HVHF/SVHF significantly decreased the number of adherent PMN/PBMC to TEC monolayers (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, TEC monolayers incubated with d3h0 plasma of HVHF/SVHF showed an enhanced adhesion of PMN/PBMC (#p < 0.05 d3h0 vs. d1h6 or d1h12). (C) FACS analysis of ICAM-1 and CD40 on TEC incubated with plasma collected at different time points of HVHF. In comparison to Vehicle alone, d1h0 plasma up-regulated ICAM-1 and CD40 expression on TEC surface (*p < 0.05 d1h0 vs. Vehicle). Incubation with plasma collected at d1h6 and d1h12 significantly decreased all adhesion molecule expression (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, d3h0 plasma newly enhanced ICAM-1 and CD40 expression (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed using HVHF or SVHF plasma.
In comparison to control healthy plasma, d1h0 plasma induced a direct pro-apoptotic effect on TEC (TUNEL assay in Fig. 5A). When TEC were incubated with d1h6 and d1h12 plasma, a significant decrease of apoptosis was detected. By contrast, a new increase of apoptotic cell death was observed using d3h0 plasma (Fig. 5A). As detected for EC, the modulation of the pro-apoptotic effect of septic plasma was similar between HVHF and SVHF. The further step was to investigate the potential mechanisms involved in TEC apoptosis: in comparison to control plasma, d1h0 plasma from HVHF patients induced a significant increase of caspase-3, -8 and -9 activities (Fig. 5B) and of expression of the death receptor Fas (Fig. 5C). A significant decrease of caspase activities (Fig. 5B) and Fas expression (Fig. 5C) was detected using d1h6 and d1h12 plasma. By contrast, incubation of TEC with d3h0 plasma resulted in a new significant increase of caspase activity and Fas expression (Fig. 5B,C). Similar results were observed when TEC were challenged with SVHF plasma (not shown). The pivotal role of the circulating inflammatory mediators TNF-α, Fas-Ligand and CD40-ligand in TEC injury was further confirmed by the significant decrease of d1h0 septic plasma-induced apoptosis in cells engineered to knock-down the corresponding surface receptors TNF-Receptor, Fas or CD40 by specific siRNAs (Fig. 6).
Evaluation of TEC apoptosis after incubation with plasma collected at different HVHF/SVHF time points. (A) Evaluation of TEC apoptosis by TUNEL assay to detect DNA fragmentation in the presence of plasma collected at different time points after HVHF (black columns) or SVHF (white columns) start. Incubation of TEC with d1h0 plasma significantly increased the number of apoptotic cells (*p < 0.05 d1h0 vs. Vehicle). In respect to d1h0 plasma, incubation of TEC with d1h6 or d1h12 plasma showed a significant decrease of apoptosis (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, d3h0 plasma newly increased the number of apoptotic TEC (#p < 0.05 d3h0 vs. d1h6 or d1h12). The number of apoptotic cells showing DNA fragmentation was counted in 30 different microscopic fields: data are shown as average ± 1SD of 3 different experiments. Similar results were observed for caspase-3 (white columns), caspase-8 (black columns) and caspase-9 (gray columns) activities (all showed in B). Graph shows ELISA results after incubation of TEC with HVHF plasma: similar results were observed using SVHF plasma. (C) Representative images showing Fas expression on TEC after incubation with HVHF plasma collected at different time points. In comparison to Vehicle alone, d1h0 plasma enhanced Fas expression. In respect to d1h0, Fas staining was lower in TEC incubated with d1h6 and d1h12 plasma. By contrast, d3h0 plasma newly increased the expression of this death receptor molecule. Three experiments were performed with similar results. Nuclei were counterstained by Hoechst in blue. Similar results were observed using SVHF plasma.
Modulation of plasma-induced TEC apoptosis after TNF-R, Fas or CD40 knock-down by siRNA. Evaluation of apoptosis by TUNEL assay in wild type TEC (Vehicle), TEC transfected with an irrelevant siRNA (siRNA control) or TEC enginereed to knock-down TNF-R, Fas or CD40 by specific siRNA. In comparison to Vehicle or siRNA control, siRNA TNF-R, siRNA Fas or siRNA CD40 TEC showed a significant decrease of apoptotic cell death (*p < 0.05 siRNA TNF-R, siRNA Fas or siRNA CD40 vs. Vehicle or siRNA Control). The number of apoptotic cells was counted in 30 different microscopic fields: data are shown as average ± 1SD of 3 different experiments.
Apart from apoptosis, we also compared the effect of HVHF vs. SVHF on loss of cell polarity, a typical functional alteration of TEC associated with de-differentiation and commonly observed during SA-AKI. In comparison to control healthy plasma, d1h0 plasma induced the loss of polarity assessed by TEER (Fig. 7A). When TEC were incubated with d1h6 and d1h12 plasma, a significant increase of TEER was detected. By contrast, a new reduction of TEER was observed using d3h0 plasma (Fig. 7A). The modulation of cell polarity induced by septic plasma was comparable between HVHF and SVHF. Similar results were observed studying albumin uptake by TEC incubated with septic plasma drawn at different time points of HVHF or SVHF (Fig. 7B). We then investigated the potential mechanisms involved in loss of TEC polarity and albumin uptake: we observed that in comparison to control plasma, d1h0 plasma from HVHF patients induced the down-regulation of the endocytic receptor megalin on TEC surface (Fig. 7C,D). The loss of megalin expression was less pronounced in the presence of d1h6 and d1h12 plasma, but not with d3h0 plasma (Fig. 7C,D). Equivalent results were observed for sodium (NKCC1) and glucose (SGLT-2) tubular transporters (Fig. 7C,D). Similar data were found when TEC were incubated with SVHF plasma (not shown).
Evaluation of TEC polarity after incubation with plasma collected at different HVHF/SVHF time points. (A, B) Evaluation of cellular polarity by analysis of trans-epithelial electrical resistance (TEER in A) and FITC-albumin reabsorption (B) of TEC incubated with plasma collected at different time points after HVHF (black columns) or SVHF (white columns) start. In respect to Vehicle alone, d1h0 plasma induced a significant decrease of TEER and albumin reabsorption (*p < 0.05 d1h0 vs. Vehicle). Plasma samples collected at d1h6 and d1h12 increased TEC polarity (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, TEC monolayers incubated with d3h0 plasma showed a new significant decrease of both TEER and albumin reabsorption (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed for HVHF and SVHF. All experiments were performed in triplicate. (C, D) Representative micrographs (C) and relative fluorescence intensity quantification (D) of Megalin (green staining), NKCC1 (green staining) and SGLT-2 (red staining) expression in TEC incubated with plasma collected at different time points after HVHF start. In respect to Vehicle alone, d1h0 plasma induced a significant decrease of Megalin, NKCC1 and SGLT-2 expression (*p < 0.05 d1h0 vs. Vehicle). Plasma samples collected at d1h6 and d1h12 increased the staining for all the proteins on TEC surface (°p < 0.05 d1h6 or d1h12 vs. d1h0). By contrast, TEC monolayers incubated with d3h0 plasma showed a new significant decrease of Megalin, NKCC1 and SGLT-2 expression (#p < 0.05 d3h0 vs. d1h6 or d1h12). Similar results were observed using SVHF plasma. All experiments were performed in triplicate. In fluorescence micrographs, nuclei were counterstained in blue by Hoechst.
Effect of ultrafiltrate fluid (UF) collected during HVHF or SVHF on EC and TEC injury
Since the removal of inflammatory cytokines during hemofiltration is supposed to be mainly due to convection-driven forces, we investigated whether ultrafiltrate fluid (UF) collected during HVHF or SVHF may modulate the observed EC and TEC damage. We first set up a dose–response experiment in which we found that the use of 50% UF diluted in 50% normal culture medium induced a 10 × increase of the number of apoptotic cells after 48 h of incubation (not shown). We then used this ratio of UF/medium for the following experiments: in comparison to vehicle (culture medium alone), UF obtained from HVHF or SVHF induced a significant increase of apoptosis of both EC (Fig. 8A) and TEC (Fig. 8B) at the different time points studied (12, 24, 48 and 72 h). Despite UF from HVHF induced a higher pro-apoptotic effect than those collected from SVHF in both cell types, no significant statistical difference was observed (Fig. 8A,B).
Assessment of EC and TEC apoptosis after incubation with HVHF or SVHF ultrafiltrate fluid. (A-B) TUNEL assay showing the number of apoptotic EC (A) and TEC (B) after incubation with HVHF or SVHF ultrafiltrates. In respect to Vehicle (culture medium alone), ultrafiltrate fluids induced a significant increase of both EC and TEC apoptosis at all time points considered (12–24–48–72 h). No significant difference was observed between HVHF and SVHF ultrafiltrates (*p < 0.05 HVHF or SVHF vs. Vehicle). The number of apoptotic cells showing DNA fragmentation was counted in 30 different microscopic fields: data are shown as average ± 1SD of 3 different experiments.
Cytokine mass adsorption during in vitro hemofiltration with increasing convective doses
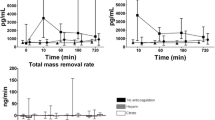
As previously described9, inflammatory cytokines can be removed not only by convection, but also by membrane adsorption. However, the polyethersulfone filter used in the IVOIRE study had low adsorption capacity: for this reason, we established an in vitro hemofiltration model using LPS-activated human blood to evaluate mass adsorption (Mad) of the same inflammatory cytokines measured in patients’ plasma (TNF-α, FasL, CD40L) in presence of increasing convective volumes (2.5 vs. 5 L/hr) to fully mimic in vitro the IVOIRE study8,12. We found that Mad of all selected cytokines were not significantly different between the HVHF and SVHF group, suggesting that increasing convective doses did not influence cytokine removal using a non-adsorptive membrane (Fig. 9A–C).
Cytokine mass adsorption (Mad) during in vitro hemofiltration with increasing convective doses. Analysis of Mass Adsorption (Mad) for TNF‐α, FasL and CD40L assessed by ELISA during in vitro hemofiltration (1, 6 and 12 h) with increasing convective doses (5L/h as HVHF vs. 2.5 L/h as SVHF) for a total duration of 12 h. No significant differences of TNF‐α, FasL and CD40L concentrations were observed between the HVHF and SVHF group at all time points considered. All experiments were performed in triplicate.
Discussion
In the present study, we confirmed previous findings4,10,11,12 showing that septic plasma collected from patients included in the IVOIRE study induced a direct injury of human kidney-derived EC and TEC, favoring leukocyte adhesion, inducing apoptosis and altering different biological functions typical of SA-AKI. These detrimental biological effects were mainly ascribed to the presence of pro-inflammatory and pro-apoptotic mediators that can be removed by EBPT. We herein observed that HVHF (70 ml/Kg/hr) and SVHF (35 ml/Kg/hr) were both able to remove the middle molecules TNF-α, FasL and CD40L from plasma independently from the convective dose used. Cytokine removal seemed to be limited at 6–12 h after the start of convective treatment using a non-adsorptive membrane and heparin as anticoagulation strategy. Moreover, functional alterations and apoptotic cell death induced by septic plasma on EC and TEC were not modulated by the increase of convective doses, since samples collected after HVHF or SVHF did not show any significant difference in several in vitro assays.
According to the Sepsis-3 consensus13, sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection and septic shock as a subset of sepsis in which severe circulatory, cellular and metabolic abnormalities are observed and often associated with a higher mortality risk than sepsis alone. Sepsis-associated AKI (SA-AKI) is defined as an abrupt renal dysfunction episode graded basing on serum creatinine levels and urinary output in accordance with the KDIGO 2012 criteria14. Recent clinical observations suggested that the association of sepsis and AKI worsens patients' outcome: the survival of SA-AKI patients is strongly correlated with the recovery of renal function15. In comparison to other causes of acute loss of renal function, SA-AKI is characterized by higher mortality rates, increased length of hospital stay and development of comorbidities including the progression from AKI to chronic kidney disease (CKD)16.
In the last years, the pathogenic mechanisms of SA-AKI have been fully elucidated: several clinical and experimental studies showed that renal damage in the course of sepsis is not merely related to tissue hypoperfusion but to mechanisms that are more immunologic or toxic in nature. As suggested by the 28th acute disease quality initiative (ADQI) workgroup, SA-AKI is a heterogeneous syndrome that occurs in correlation with both host response to infection and sepsis therapies, thus identifying sepsis-induced AKI (SI-AKI) as a subphenotype of SA-AKI, in which sepsis is the only direct driver of kidney damage17. Our group has previously demonstrated that septic plasma contains circulating pro-inflammatory and pro-apoptotic factors able to induce a direct damage of peritubular endothelial cells (EC) and tubular epithelial cells (TEC)4,10,11,12. This is in accordance with the hypothesis that circulating pathogen-associated molecular pattern (PAMPs) and damage-associated molecular pattern (DAMPs) molecules can alter TEC metabolic functions18. Other studies suggested that in SA-AKI, metabolic reprogramming and tolerance are key elements to determine the extent of organ dysfunction and the development of maladaptive repair leading to early fibrosis and CKD progression19. In the present study, we confirmed that incubation with septic plasma led to a pro-inflammatory phenotype in both EC and TEC as assessed by the increased binding of neutrophils and monocytes and by the up-regulation of adhesion molecules on cell surface. Moreover, prolonged incubation with septic plasma triggered apoptosis and inhibited angiogenesis in EC and induced in TEC loss of polarity and functional alterations.
It has been previously shown that elevated plasma concentrations of inflammatory and apoptosis biomarkers are associated with an increased risk of death and start of renal replacement therapy (RRT) in septic patients20. AKI can worsen this inflammatory response, since EC and TEC are immunologically active cells able to present antigens and to modulate cytokine levels through secretion and re-absorption mechanisms21. In this paper, we focused on TNF‐α, FasL and CD40L because these molecules can directly interact with specific receptors located on TEC causing several alterations of cellular functions12. Moreover, plasma levels of FasL and CD40L are associated with severity of sepsis and are predictors of mortality22,23. TNF‐α and FasL are also key mediators of LPS-induced AKI, acting through binding to TNFR and Fas located on TEC and triggering receptor-mediated apoptotic cell death24. The activation of the CD40/CD40L pathway has been shown to enhance tubulo-interstitial inflammation in different experimental models25,26. The role of these cytokines in SA-AKI was further confirmed in the present study in which we demonstrated that in respect to wild-type TEC, the incubation of cells previously engineered to knock-down TNF-R, Fas or CD40 with plasma collected at T0 of the IVOIRE study showed a significant reduction of apoptosis and functional alterations.
According to the “peak concentration hypothesis” proposed by Ronco et al.27, EBPT may modulate cytokine concentrations through different physico-chemical mechanisms27. In the landmark “Vicenza study”, a higher convective dose was associated with a better survival28: however, later clinical trials including the RENAL29 and the ATN30 studies did not confirm these promising results. Also in the IVOIRE study, HVHF (70 mL/kg/h) did not show a survival and clinical benefit in respect to SVHF (35 mL/kg/h): in addition, HVHF may lead to nutrient, vitamin and trace-metal depletion as well as to a subtherapeutic concentration of antibiotics8,31. In this translational study using samples from the IVOIRE trial, we observed that the decrease of plasma levels of TNF‐α, FasL and CD40L as well as of the biomarker of endothelial damage vWF was time-limited, since all these mediators decreased from the start to 12 h of hemofiltration without any significant difference between HVHF vs SVHF. Moreover, cytokine levels newly increased from 12 to 48 h when the filter was changed. The modulation of cytokine levels was comparable to the biological effects exerted by patients’ plasma on EC and TEC: we noticed that in comparison to plasma collected at the start of both HVHF and SVHF, those gathered after 6 h or 12 h induced a significant decrease of inflammation and angiogenesis inhibition on EC and of inflammation, apoptosis and loss of cell polarity on TEC. These protective effects were blunted after 12 h of treatment without any significant difference between HVHF and SVHF. Furthermore, the percentage of CD31 + endothelial-derived microparticles remained stable for all the treatment time without any significant difference between HVHF and SVHF: these results suggested the presence of a persistent microvascular injury in enrolled patients and that endothelial microparticles are not removed by convection.
These results were confirmed by in vitro hemofiltration using LPS-activated blood and by in vitro experiments using ultrafiltrates collected from the IVOIRE patients on EC and TEC: mass removals of TNF‐α, FasL and CD40L were not influenced by increasing the convective dose during in vitro hemofiltration. In addition, in respect to SVHF, HVHF was not correlated with an increased concentration of cytokines in the ultrafiltrate fluid. For this reason, we did not observe any difference in EC and TEC injury incubated with ultrafiltrates collected at different time points after HVHF or SVHF.
Based on the above-described results, our main working hypothesis is that the increase of convective dose during hemofiltration using a non-adsorptive membrane and heparin as anticoagulation strategy is not able to significantly modulate cytokine plasma levels. A possible alternative strategy may be represented by using regional citrate anticoagulation (RCA) that was not adopted in the IVOIRE study. Indeed. several RCTs including the recent RICH study32 demonstrated that RCA is able to significantly increase filter lifespan without any effect on mortality. One could speculate that RCA could increase cytokine and uremic toxin clearances due to its enhanced ability to avoid filter clotting. Another interesting consideration is that none of the RCTs studying the effect of dose on mortality including IVOIRE utilized a membrane with adsorptive properties. Using the same methodology that we adopted in the present study to evaluate cytokine mass transfer, De Vriese and coworkers previously demonstrated that during hemofiltration using AN69 membrane in SA-AKI patients, cytokine removal was mainly ascribed to adsorption rather than convection9. Following experimental and clinical studies confirmed the capacity of AN69-PEI-heparin and polymethylmethacrylate (PMMA) membranes to adsorb inflammatory cytokines. Our research group recently demonstrated in an experimental pig model of sepsis that PMMA-based hemofiltration can modulate immunologic dysfunction and renal damage33.
We acknowledge that this study presents some weaknesses: first, due to technological difficulties and to the limited volume of samples, the in vitro studies on EC and TEC were performed with plasma collected from a small number of patients enrolled in both HVHF and SVHF groups. The use of heparin as only anticoagulant strategy may influence the inflammatory response and filter clearance of different molecules: moreover, the levels of antithrombin, the necessary cofactor for heparin activity, were not determined. Last, cytokine levels were measured only in plasma and not in the ultrafiltrate fluid collected during HVHF and SVHF and used for in vitro studies: the lack of cytokine concentrations in the ultrafiltrate collected from patients did not allow the determination of mass adsorption of the different inflammatory mediators, likewise we performed in the in vitro hemofiltration model in accordance to De Vriese et al.9.
This study also presents some strengths: first, in vitro studies were performed using plasma collected from SA-AKI patients enrolled in the IVOIRE study representing an adequate sample of the ICU population currently treated by EBPT. The modulation of cytokine levels observed in patients was correlated with several in vitro assays aimed to study EC and TEC damage and functional alterations typical of SA-AKI. Last, the results of the present study confirmed previous in vitro observations on septic plasma-induced renal cell injury3,4,10.
Conclusions
When compared to SVHF, HVHF did not increase the clearance of inflammatory cytokines and did not reverse the detrimental effects of septic plasma-induced EC and TEC injury. Further experimental studies and clinical trials using adsorptive membranes/sorbents and/or citrate as anticoagulation strategy are needed to evaluate the potential role of high convection doses in the limitation of the harmful activity of plasma soluble factors involved in SA-AKI.
Methods
Selection of patients and renal replacement therapies (RRT)
Ten patients from the IVOIRE trial (hIgh VOlume in Intensive caRE) were enrolled in the present study. IVOIRE was a prospective randomized clinical trial conducted in 18 ICUs in France, Belgium and the Netherlands with the primary objective to evaluate 28-day mortality in critically ill patients with septic shock and AKI subjected to high-volume hemofiltration (HVHF, 70 mL/kg/hr), compared with standard-volume hemofiltration (SVHF, 35 mL/kg/hr) for 96 consecutive hrs8. Ethics approval was obtained from the Human Research Ethics Committee for South-West France and Overseas Departments (CPP SOOM III, approval number 05–01), from each participating institution’s ethics committee for centers outside France or from the French National Agency for the Safety of Health Products (AFSSAPS). Written informed consent was obtained from each participant or next of kin. All methods were performed in accordance with regulations for clinical trials and the Declaration of Helsinki. The trial protocol was registered on ClinicalTrials.gov (identifier: NCT00241228; start date 18/10/2005: web link: https://clinicaltrials.gov/study/NCT00241228?term=NCT00241228&rank=1)34. The inclusion criteria were: age ≥ 18 years, presence of septic shock defined according to the consensus definition of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee13, for a period of less than 24 h, and presence of AKI with inclusion at least in the INJURY category of the RIFLE classification by either serum creatinine and/or urine output criteria35. Exclusion criteria were: age > 80 years, estimated life expectancy ≤ 3 months, metastatic cancer, decompensated hepatic cirrhosis, acute necrotizing pancreatitis, previous diagnosis of end stage chronic kidney disease, pregnancy, severe coagulopathy (INR > 3 and/or platelet count < 20,000 cells/mL) and lack of consent. All patients received standard therapies for septic shock in accordance with the best contemporary practice and consensus guideline recommendations8. After randomization, RRT was performed with Aquarius® hemofiltration circuit (Edwards Lifesciences Corp., Irvine, CA) equipped with a 1.9 m2 Aquamax® polyethersulfone filter (Edwards Lifesciences; molecular weight cut-off approximately 35 KDa; low adsorption capacity). In the HVHF and SVHF group, hemofiltration was prescribed at a dose of 70 or 35 mL/kg/hr for 96 h, respectively. In both groups, blood flow rates were modified to provide a filtration fraction ≤ 25% with a target transmembrane pressure maintained between 100 and 300 mmHg and using an average 33% predilution. Circuit anticoagulation was performed using unfractioned heparin and ultrafiltration rate was adapted to each patient need. Filter change was performed every 48 h8. Plasma and ultrafiltration fluid from 5 patients of the HVHF and 5 of the SVHF group were sterilely collected at different time points directly from the sampling sites of the circuit and then used for cytokine measurements and in vitro studies: the main clinical characteristics of enrolled patients are reported in Table 1.
As internal experimental control for the in vitro studies described in the next paragraphs, a healthy population (n = 10) matched for age, sex and comorbidities was selected: the average age of healthy controls was 69.4 ± 2.26 (IVOIRE group: 68.8 ± 2.08; SVHF group 67.8 ± 1.16 and HVHF group 69.8 ± 2.31, respectively); sex distribution was 70% males and the prevalence of diabetes was 20% in both groups.
Plasma analyses
Blood samples during SVHF and HVHF were collected at the start (d1h0) and after 6 (d1h6), 12 (d1h12) and 48 h (d3ho), respectively. Plasma was stored at − 80 °C until use: all samples were used for laboratory studies no longer than 12 months from storage. Plasma levels of TNF-alpha, Fas-Ligand (FasL), CD40-Ligand (CD40L) and von Willlebrand factor (vWF) were determined by commercially available ELISA (TNF-alpha, FasL, CD40L ELISA were obtained by R&D Systems, Minneapolis, MN, USA; vWF ELISA was obtained by Affinity Biologicals, Inc. Ancaster, ON, Canada). Endothelial microparticles were evaluated as percentage of total plasma extracellular vesicles by Nanosight determination and FACS using selected beads directed to the endothelial antigen CD3136.
In vitro studies on human endothelial (EC) and tubular epithelial cells (TEC)
Human endothelial EC and TEC were isolated from kidneys removed by surgical procedures from patients affected by renal carcinomas and characterized as previously described37,38.
Adhesion of inflammatory cells to EC and TEC
Polymorphonuclear neutrophils (PMN) and peripheral blood mononuclear cells (PBMC) were isolated from blood of healthy volunteers by density centrifugation as previously described37,38 and labeled overnight with 10 μM Vybrant Cell Tracer kit (Invitrogen, San Diego, CA, USA) according to manufacturer’s instructions. Labeled cells were counted, resuspended to 50 × 106/ml RPMI and added to a confluent monolayer of EC or TEC cultured on six-well plates and previously incubated with different plasma samples. Experiments were carried out in triplicate for 1 h. at 37 °C in conditions of slight agitation. At the end of incubation, plates were filled with medium and aspirated thrice to remove unbound cells. All samples were fixed with 4% paraformaldehyde and observed under a UV light microscope. Green fluorescent cells were counted on 10 different fields at × 100 magnification.
Cytotoxicity and apoptosis
EC or TEC were cultured on 24-well plates (Falcon Labware, Oxnard, CA, USA) at a concentration of 5 × 104 cells/well and incubated with different plasma concentrations and 250 μg/ml XTT (Sigma, St. Louis, MO, USA) in a medium lacking phenol red. The absorption values at 450 nm were measured in an automated spectrophotometer at different time points. All experiments were performed in triplicate. For detection of apoptosis by TUNEL assay, EC and TEC were incubated for 48 h with 10% plasma collected at different time points from the start of hemofiltration, fixed with 4% paraformaldehyde and then subjected to terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) assay (ApopTag, Oncor, Gaithersburg, MD, USA) that identifies DNA fragmentation, a typical feature of apoptotic cells. Green-stained apoptotic cells were counted in different microscopic fields at × 100 magnification. The activity of caspase-3, -8 and -9 was assessed only in TEC by a colorimetric assay (Chemicon Int., Temecula, CA, USA) based on the spectrophotometric detection of the cromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA by caspases37. Each experiment was performed in triplicate. Results are given as average of percentage increase of caspase activity in respect to incubation with control healthy plasma ± 1SD.
Trans-epithelial electrical resistance (TEER)
Trans-epithelial electrical resistance (TEER) was used as an indicator of TEC polarity. Cells were plated in transwells on collagen-coated polycarbonate membranes (Corning Costar Corp., Cambridge, MA, USA) and allowed to reach confluence before the addition of different plasma samples. An epithelial volt-ohm meter (EVOM; World Precision Instruments, Inc., Sarasota, FL, USA) was used to determine TEER values as previously described3. All measures were performed in triplicate and normalized for the area of the membrane.
In vitro endothelial cell angiogenesis and tubular morphogenesis assays
The formation of capillary-like structures was studied on EC (5 × 104 cells/well) seeded on growth factor–reduced Matrigel (Becton Dickinson) diluted 1:1 in ice with cold Dulbecco Modified Eagle Medium (DMEM; Sigma-Aldrich). After cells had attached, 1 mL medium containing different plasma (10% dilution) was added. Cells were observed under a Nikon-inverted microscope (Kanagawa, Japan), using a 10 × /0.25 NA objective lens, and experimental results were recorded after 6-h incubation at 37 °C. For morphogenesis studies, TEC were cultured on Matrigel-coated plates for 72 h and in the presence of different plasma for 6 h at 37 °C. Image analysis was performed with the MicroImage analysis system (Casti Imaging, Venice, Italy)39.
Detection of FITC-conjugated albumin uptake by TEC
Albumin uptake was studied after incubation of TEC with different plasma for 12 h and then with 50 mg/ml of FITC-conjugated human albumin (Sigma, St. Louis, MO, USA) at 37 °C for 2 h: after FITC-albumin challenge, TEC were extensively washed twice with cold 1 × PBS and analyzed by FACS12.
Immunofluorescence and FACS studies
After appropriate stimuli, cultured TEC were fixed in ethanol/acetic acid 2:1 and stained with antibodies directed to human Fas (Upstate Biotechnology, Lake Placid, NY, USA), megalin, NKCC1 or SGLT-2 (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with primary antibodies, samples were washed twice with 1 × PBS and incubated with appropriated Alexa Fluor-conjugated secondary antibodies (Molecular Probes, Carlsbad, CA, USA) for 30 min, room temperature when needed. All samples were counterstained by 1 μg/ml propidium iodide mounted with anti-fade mounting medium (Vector Laboratories, Burlingame, CA, USA) and examined by confocal microscopy. For FACS analysis, after exposure to different plasmas, EC or TEC were detached from tissue culture plates with EDTA, washed twice with 1 × PBS and stained for 1 h at 4 °C with FITC-conjugated antibodies directed to human Fas, CD40, inter-cellular adhesion molecule-1 (ICAM-1), vascular-cell adhesion molecule-1 (VCAM-1), E-selectin or with an irrelevant control antibody16. All incubation periods were performed using a medium containing 0.25% BSA and 0.0016% sodium azide. At the end of staining, cells were newly washed, fixed in 4% paraformaldehyde and subjected to FACS analysis (Becton Dickinson).
RNA Interference on TEC
In selected experiments, TEC were seeded on 24-well plates and engineered to knock-down TNF-Receptor, Fas or CD40, by transfection with 80 pM of specific siRNAs. Transfection with 80 pM of irrelevant siRNA was used as experimental control. After 48 h, the knock-down of TNF-R, Fas or CD40 was verified by qRT-PCR, western blot and FACS analysis and cells were incubated with different stimuli and used for TUNEL assays as previously published by our group12,37.
In vitro hemofiltration and assessment of cytokine mass adsorption
This in vitro model was designed to replicate the clinical setting of SVHF and HVHF used in the IVOIRE study: briefly, the extracorporeal circuit was a closed loop, post-dilutional hemofiltration circuit with 3 sampling sites (arterial site: before the filter; venous site: immediately after the filter and before the ultrafiltrate reinfusion site; ultrafiltrate site: on the ultrafiltrate line). For the purpose of this experiment, whole blood was treated with 1 mg bacterial lipopolysaccharide (LPS) for 4 h in a water bath at 37 °C and then overnight at room temperature. The day after, blood was heparinized (5000 U), recalcified (calcium chloride 10%, 44 μl/ml blood) and diluted 1:1 with isotonic saline solution before the use for in vitro hemofiltration. According to the IVOIRE trial8, the membrane used was the 1.9 m2 Aquamax® polyethersulfone filter (Edwards Lifesciences): circuit anticoagulation was performed with unfractioned heparin (2500 U initial bolus and subsequent 1000 U/hr). To simulate the IVOIRE study in vitro, we adopted 2 different convective doses (5 L/h vs. 2.5L/h of sterile fluid reinfusate to mimic HVHF or SVHF, respectively). In vitro hemofiltration was performed for 12 h and samples were collected from arterial, venous and ultrafiltrate sites at different time points (0–6–12 h) during treatment and stored at − 80 °C until use. Mass balance of different cytokines at each time point was calculated by ELISA for TNF-α, CD40L and FasL as previously described9. The calculation of cytokine Mass Transfer (Mtr) and mass adsorption (Mad) was obtained using the following parameters:
where Qin is inlet plasma flow rate (ml/min); Qout is outlet plasma flow rate (ml/min); Quf is ultrafiltrate flow rate (ml/min); Cin is concentration in inlet plasma (pg/ml); Cout is concentration in outlet plasma (pg/ml); Cuf is concentration in ultrafiltration fluid (pg/ml); Min is inlet mass rate (pg/min); Mout is outlet mass rate (pg/min); Muf is mass removal rate by ultrafiltration (pg/min); Mtr is total mass transfer (pg/min); Mad is mass removal rate by membrane adsorption (pg/min), SC is the sieving coefficient.
Statistical analysis
All data of different experimental procedures are expressed as average ± 1SD. Statistical analysis was performed by analysis of variance and multiple comparison with ANOVA and Newmann–Keuls multicomparison test or Student’s t-test where appropriated. For the evaluation of cytokine plasma concentrations, ANOVA and a Wilcoxon signed rank test were used. Total cytokine removal was analyzed with a Wilcoxon signed rank test. P values < 0.05 were considered as the threshold for statistical significance.
Ethics approval and consent to participate
This is a translational study associated with the IVOIRE (hIgh VOlume in Intensive caRE) randomized clinical trial. Written informed consent was obtained from each participant or next of kin. The trial protocol was registered on 18/10/2005 in ClinicalTrials.gov. IVOIRE was a prospective randomized clinical trial conducted in 18 ICUs in France, Belgium and the Netherlands with the primary objective to evaluate 28-day mortality in critically ill patients with septic shock and AKI subjected to high-volume hemofiltration (HVHF, 70 mL/kg/hr), compared with standard-volume hemofiltration (SVHF, 35 mL/kg/hr) for 96 consecutive hr.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Vijayan, A. Tackling AKI: Prevention, timing of dialysis and follow-up. Nat. Rev. Nephrol. 17(2), 87–88 (2021).
Cantaluppi, V. et al. Perfluorocarbon solutions limit tubular epithelial cell injury and promote CD133+ kidney progenitor differentiation: Potential use in renal assist devices for sepsis-associated acute kidney injury and multiple organ failure. Nephrol. Dial. Transpl. 33(7), 1110–1121 (2018).
Mariano, F. et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit. Care 12(2), R42 (2008).
Medica, D. et al. Extracellular vesicles derived from endothelial progenitor cells protect human glomerular endothelial cells and podocytes from complement- and cytokine-mediated injury. Cells 10(7), 1675 (2021).
Monard, C., Abraham, P., Schneider, A. & Rimmele’, T. New targets for extracorporeal blood purification therapies in sepsis. Blood Purif. 52(1), 1–7 (2023).
Honore, P. M. et al. Cytokine removal in human septic shock: Where are we and where are we going?. Ann. Intensive Care 9(1), 56 (2019).
Joannes-Boyau, O. et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med. 39(9), 1535–1546 (2013).
De Vriese, A. S. et al. Cytokine removal during continuous hemofiltration in septic patients. J. Am. Soc. Nephrol. 10(4), 846–853 (1999).
Cantaluppi, V. et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 34(9), 1638–1645 (2008).
Zarbock, A. et al. Sepsis-associated acute kidney injury: Consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 19(6), 401–417 (2023).
Cantaluppi, V. et al. Protective effect of resin adsorption on septic plasma-induced tubular injury. Crit. Care 14(1), R4 (2010).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Palevsky, P. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61(5), 649–672 (2013).
Fiorentino, M. et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 13(6), e0198269 (2018).
Wang, Z. & Zhang, C. From AKI to CKD: Maladaptive repair and the underlying mechanisms. Int. J. Mol. Sci. 23(18), 10880 (2022).
Vaara, S. T. et al. Subphenotypes in acute kidney injury: A narrative review. Crit. Care 26(1), 251 (2022).
Fani, F. et al. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J. Nephrol. 31(3), 351–359 (2018).
Gómez, H. & Kellum, J. A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 22(6), 546–553 (2016).
Murugan, R. et al. Plasma inflammatory and apoptosis markers are associated with dialysis dependence and death among critically ill patients receiving renal replacement therapy. Nephrol. Dial. Transplant. 29(10), 1854–1864 (2014).
Zager, R. A., Johnson, A. C. M. & Lund, S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am. J. Physiol. Renal. Physiol. 297(4), F961–F970 (2009).
Yoo, H., Lee, J. Y., Park, J., Suh, G. Y. & Jeon, K. Association of plasma levels of fas ligand with severity and outcome of sepsis. Shock 56(4), 544–550 (2021).
Lorente, L. et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit. Care 15(2), R97 (2011).
Adachi, T., Sugiyama, N., Yagita, H. & Yokoyama, T. Renal atrophy after ischemia-reperfusion injury depends on massive tubular apoptosis induced by TNFα in the later phase. Med. Mol. Morphol. 47(4), 213–223 (2014).
van Kooten, C., Woltman, A. M. & Daha, M. R. Immunological function of tubular epithelial cells: The functional implications of CD40 expression. Exp. Nephrol. 8(4–5), 203–207 (2000).
Marengo, M. et al. Role of the CD40-CD40 ligand pathway in cardiovascular events, neurological alterations, and other clinical complications of chronic hemodialysis patients: Protective role of adsorptive membranes. Blood Purif. 16, 1–16 (2023).
Ronco, C. et al. Extracorporeal therapies in non-renal disease: Treatment of sepsis and the peak concentration hypothesis. Blood Purif. 22(1), 164–174 (2004).
Ronco, C. et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: A prospective randomised trial. Lancet 356(9223), 26–30 (2000).
Finfer, S. et al. The RENAL (Randomised evaluation of normal vs. augmented level of replacement therapy) study: Statistical analysis plan. Crit. Care Resusc. 11(1), 58–66 (2009).
Palevsky, P. M. et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359(1), 7–20 (2008).
Schneider, A. G. et al. Amino acids and vitamins status during continuous renal replacement therapy: An ancillary prospective observational study of a randomised control trial. Anaesth. Crit. Care Pain. Med. 40(2), 100813 (2021).
Meersch, M. et al. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill patients with acute kidney injury (RICH) trial: Study protocol for a multicentre, randomised controlled trial. BMJ Open. 9(1), e024411 (2019).
Stasi, A. et al. PMMA-based continuous hemofiltration modulated complement activation and renal dysfunction in LPS-induced acute kidney injury. Front. Immunol. 12, 605212 (2021).
ClinicalTrials.gov: ID NCT00241228. Haemofiltration Study: IVOIRE (hIgh VOlume in Intensive Care) (IVOIRE). Information provided by University Hospital, Bordeaux. Last Update Posted 2010-10-13.
Ostermann, M. et al. Controversies in acute kidney injury: Conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int. 98(2), 294–309 (2020).
Cavallari, C. et al. Online hemodiafiltration inhibits inflammation-related endothelial dysfunction and vascular calcification of uremic patients modulating miR-223 expression in plasma extracellular vesicles. J. Immunol. 202(8), 2372–2383 (2019).
Cantaluppi, V. et al. Macrophage stimulating protein may promote tubular regeneration after acute injury. J. Am. Soc. Nephrol. 19(10), 1904–1918 (2008).
Burger, D. et al. Human endothelial colony-forming cells protect against acute kidney injury: Role of exosomes. Am. J. Pathol. 185(8), 2309–2323 (2015).
Deregibus, M. C. et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110(7), 2440–2448 (2007).
Acknowledgements
ADQ, MaMa, GiuCa, PF, MaMi and VC are members of the AKI and CRRT Project Group of the Italian Society of Nephrology (SIN). The authors thank Mr. Paolo Besati and Mr. Francesco Campanella from the Italian Association of Hemodialysis Technicians (ANTE) for their technical assistance for in vitro hemofiltration studies. Part of these results were presented and were awarded as best abstracts at the 2010 ERA-EDTA Congress in Munich (Germany), at the 2011 AKI & CRRT Congress in San Diego (CA) and at the 2011 American Society for Artificial Internal Organs (ASAIO) annual Congress in Washington DC.
Funding
Part of the present study was funded by the Italian Ministry of Education, University and Research (MIUR) program “Departments of Excellence on Aging 2018–2022” to the Department of Translational Medicine (DIMET), University of Piemonte Orientale (UPO) and by local university grants (FAR) both to VC.
Author information
Authors and Affiliations
Contributions
DM and ADQ analyzed and interpreted the effects of septic plasma collected from HVHF and SVHF septic patients on EC and TEC. They conceived the experiments, wrote the article and analyzed data. MaMa, VF, PF and GiuCa: analyzed and interpreted patient data. MaMi and GM: statistical elaboration of data and table and figure creation, wrote the article and analyzed data. OJB, PMH and VC conceived the study, supervised the experiments, wrote the article, analyzed data and approved the final version of the manuscript together with GioCa. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Medica, D., Quercia, A.D., Marengo, M. et al. High-volume hemofiltration does not protect human kidney endothelial and tubular epithelial cells from septic plasma-induced injury. Sci Rep 14, 18323 (2024). https://doi.org/10.1038/s41598-024-69202-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69202-z
- Springer Nature Limited