Abstract
This study aimed to investigate the expression and significance of insulin-like growth factor binding protein 5 (IGFBP5) and extracellular matrix (ECM) related proteins in anterior vaginal wall tissues among aged pelvic organ prolapse (POP) patients. Tissues from the anterior vaginal wall were collected from 28 patients with POP and 20 patients without POP. The expression of protein and mRNA levels of IGFBP5 and ECM related proteins were evaluated in the vaginal wall tissues using immunohistochemistry, western blotting, and RT-qPCR techniques. The expression levels were then compared with clinical parameters. The expression levels of protein and mRNA of IGFBP5, collagen I, and collagen III were significantly lower in the POP group. Protein and mRNA expression levels of MMP2 were significantly higher in the POP group. IGFBP5 protein and mRNA expression levels were negatively correlated with age and significantly lower in older POP patients (≥ 65 years old) compared to younger POP patients (< 65 years old). IGFBP5 protein and mRNA expression levels were also significantly lower in POP-Q stage IV patients compared to POP-Q stage III patients. IGFBP5 expression level is negatively correlated with the age and severity of prolapse. The significant decrease in IGFBP5 expression may play a crucial part in the aging process and the occurrence of POP.
Similar content being viewed by others
Introduction
Pelvic organ prolapse (POP) is a common and chronic disease characterized by the weakness of the supportive structure1,2,3. The pelvic organs are maintained in proper anatomical position via a complex supporting structural system that is mainly composed of connective tissue. Connective tissue is composed of fibroblasts and their secreted extracellular matrix (ECM). Fibroblasts secreted and released collagen, contributing to the stability and adaptability of the pelvic floor. The degradation of collagen is carried out by a category of proteolytic enzymes known as matrix metalloproteinases (MMPs), while the function of MMPs is hindered by a category of proteins called tissue inhibitors of metalloproteins (TIMPs). Recent researches suggest that changes in the connective tissue can cause damage to the pelvic floor support structures, thus contributing to the occurrence of POP4,5. Already identified factors of POP include but are not limited to advanced age, menopause, obesity, pregnancy, vaginal delivery, estrogen deficiency, constipation, heavy lifting, chronic cough, and diabetes6. These factors may contribute to abnormal metabolism and remodeling of ECM in the pelvic floor, which subsequently affect their mechanical properties. Although there have been many researches on POP, little is known about its exact etiology and pathogenesis. Early prevention and treatment can be achieved through further research on the pathogenesis of pelvic organ prolapse.
Insulin-like growth factor binding proteins (IGFBPs) are secretory proteins that bind to IGFs or other ligands, and the IGFBPs exert both IGF-dependent and -independent effects7. IGFBP5 is the most conserved among the six IGFBPs and exhibits extensive biological activities including promoting cell survival, proliferation, senescence, differentiation, and apoptosis8. The localization of IGFBP5 within or outside of cells, specificity of the cell and tissue environment can influence the function of IGFBP59. The levels of IGFBP5 expression were lower in aged adults than in young adults in the serum, bone, and skeletal muscle10,11. Contrary to the downregulation of IGFBP5 due to aging, the upregulated expression of IGFBP5 is observed in both human and mouse aging thymus12. A study has indicated that IGFBP5 can facilitate cell proliferation or apoptosis when cells undergo different contexts and conditions13. Nguyen’s findings suggested that IGFBP5 is both an anti-angiogenic and pro-fibrotic factor7. IGFBP5 also plays a crucial role in regulating fibrosis and inflammation in various tissues14. IGFBP5 expression considerably increases and contribute to extracellular matrix deposition in fibrotic diseases, such as idiopathic pulmonary fibrosis and systemic sclerosis15. One study also confirmed that mice expressing human IGFBP-5 exhibited increased ECM gene expression to promote fibrosis16. Previous reports have examined the expression and biological activities of IGFBP5 in various tissues, however, there has been no research on exploring the relationships between IGFBP5 and POP.
IGFBP5 is associated with senescence17 and regulating fibrosis. In this study, we hypothesis that the decrease of IGFBP5 expression plays a crucial role in POP. The major objective of this research was to estimate the expression level of IGFBP5 in the vaginal wall among patients suffering from POP and explore weather IGFBP5 influences the occurrence and development of POP.
Materials and methods
Patient selection
Our investigation was performed following the Declaration of Helsinki. The protocol with code 2022PS154K was approved by the ethics committee of Shengjing Hospital of China Medical University. All the patients collected in a blinded, randomized fashion from February 2022 to August 2023. This study included 28 patients had advanced POP (stage III or IV in accordance with the POP-Q system) diagnosed by the same experienced expert and underwent pelvic floor reconstruction surgery, 20 patients without POP who had benign gynecological diseases and underwent total hysterectomy surgery. In order to rule out the impact of these diseases on the findings of this experimental, patients who had previous history of pelvic floor reconstruction surgery, connective tissue disorders, pelvic inflammatory conditions, hormone replacement therapy (HRT), gynecologic malignancies, endometriosis, and emphysema were excluded. 20 (aged 39–68 years) of the 28 (aged 39–82 years) POP patients were selected for the POP group and 20 (aged 48–73 years) patients without POP in the control group.
Tissue collection
During surgery, collected full-thickness vaginal wall tissue (approximately 0.5–1 cm2) from the obviously prolapsed region of the anterior vaginal wall near the vaginal apex of the POP patients and anterior vaginal wall near the vaginal apex of the control patients. Each specimen was divided into pieces. One piece was treated with formalin and embedded in 10% neutral-buffered paraffin for Masson's trichrome, HE, and immunohistochemical analyses. While the other piece was stored at – 80 °C for RNA and protein analyses. Sometimes tissue was used only for one purpose due to some variability in the size of the sample.
Hematoxylin and eosin (HE) and Masson’s trichrome staining
The vaginal specimens from both POP and control groups were sectioned longitudinally and put in 10% neutral buffered formalin for 2 days. They were cut into slides that were 4 μm thick, and placed onto coated slides after embedded in paraffin. The slides were subjected to the temperature at 60 °C for 120 min, deparaffinized, and were gradually rehydrated utilizing different concentrations of alcohol. They were then stained with HE and Masson’s trichrome kit (Coolaber, SL7230, Beijing, China).
Immunohistochemistry
The UltraSensitive SP IHC kit (Maixin Biotech, Kit-9710, Fuzhou, China), was employed to conduct immunochemistry. After heating for120 min at a temperature of 60 °C, deparaffinized, and rehydrated in gradient concentration alcohol. The slides were boiled in EDTA buffer for antigen retrieval in accordance with manufacturer. At room temperature, the sections were incubated for 10 min with 3% H2O2, blocked for 10 min with 5% goat serum. Subsequently, incubated with primary antibodies overnight at 4 °C. Next, sections were incubated for 10 min with biotinylated secondary antibodies at room temperature, and incubated for 10 min with streptavidin peroxidase at room temperature. After each step, specimens underwent PBS washing. Immune reaction was visualized using the DAB-ZLI-9017 kit (ZSGB-Biotech, Beijing, China). Finally, the slides underwent water washing prior to applying hematoxylin as a counterstain. Negative controls were treated with PBS instead of the primary antibody. The antibodies used are shown in Supplementary Table 1. Images were obtained utilizing a Nikon microscope (Nikon E100 Optical, Tokyo, Japan). The slides were examined using light microscopy at a magnification of 400×, maintaining the same camera settings. Selected five regions for every slide randomly. The positive area percentage and staining intensity were analyzed in all regions of the tissue (mean density = IOD sum/area sum) using ImageJ software (NIH, Bethesda, MD, USA). The average value of the selected five regions was utilized to quantify the level of target protein on each slide. Regions containing vessels were excluded from the analysis.
Western blotting
Cutting off the mucosal and muscular layers of anterior vaginal wall, total proteins were extracted by lysing the lamina propria tissue of vaginal samples with RIPA lysis buffer (Seven Biotech, SW104, Beijing, China) as instructed by the manufacturer. Measured the proteins utilizing the Omni-Easy™ Instant BCA Protein Assay Kit (Epizyme Biomedical Technology, ZJ102, Shanghai, China). Then, same amounts of protein from each sample were separated after subjected to SDS- PAGE. The separated proteins were transferred onto PVDF membranes using a current of 200 mA at 4 °C for 1–3 h. The membranes were blocked with a solution containing 5% non-fat milk at room temperature for 2 h. Subsequently, the membranes were incubated with primary antibodies at 4 °C overnight. The primary antibodies used are shown in Supplementary Table 2. Afterwards, the membranes were incubated with goat anti-rabbit secondary antibodies (1:3000, SA00001-15, Proteintech, USA) at room temperature for 1 h. After each antibody incubation step, unbound antibodies underwent TBST washing five times. The enhanced chemiluminescence (ECL) (MCE, Shanghai, China) detection method was used to visualize target bands. Beta actin served as an internal control. ImageJ software was employed to determine the density of all bands. A proportion of the target protein to the internal reference protein was used to express values of target protein.
Reverse transcription and quantitative real‐time PCR
Total tissue RNA was obtained from the lamina propria of the anterior vaginal wall using TRIzol reagent (Seven Biotech, SM139-02, Beijing, China) as instructed by the manufacturer. The quality and quantity of total RNA were determined making use of the Nano-300 (All SHENG, Hangzhou, China) and cDNA synthesis was carried out using a reverse transcription kit (TransGen Biotech, AU341, Beijing, China) (1 µg of total RNA and in a reaction volume of 20 µl): 55 °C for 5 min, 85 °C for 5 s, then remained at 4 °C. RT-qPCR assays were performed on an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) making use of the PerfectStart® Green qPCR SuperMix kit (TransGen Biotech, AQ601, Beijing, China). Sangon Biotech (Shanghai, China) synthesized the primers. Primer sequences are provided in Table 1. The reactions were conducted in triplicate. The reactions followed a protocol of 30 s at 94 °C, followed by 40 cycles of 5 s at 94 °C and 34 s at 60 °C, with a melting curve in the end. Data was analyzed using the relative quantitative method and GAPDH was used as the internal control. Three replicates being performed for each sample. The relative expression of each target gene was determined using 2−ΔΔCt method.
Statistical analysis
The statistical software SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was utilized for conducting the statistical analysis. Data were analyzed with independent sample t-test for continuous data with normal distributions, Mann–Whitney test for data with non-normal distributions, and chi‐squared test for categorical data. Normal continuous variables were expressed as mean ± standard deviation (SD), while non-normal continuous variables were reported as median (interquartile range). Categorical variables were presented as numbers and proportions. GraphPad Prism 9.3.0 (GraphPad Software, Inc., La Jolla, CA, USA) was utilized for graphical plotting. Variations were regarded as statistically significant when P < 0.05.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shengjing Hospital of China Medical University (No. 2022PS154K).
Consent to participate
Informed consent is not necessary to obtain in this study because the tissues are abandoned in surgery the informed consent is exempted by the ethics committee of Shengjing Hospital of China Medical University.
Results
Baseline characteristics of patients
In Table 2, the baseline characteristics of the patients in control group and POP group are showed. No noteworthy disparities were noted between the two groups in the way of age, body mass index (BMI), menopausal status, cesarean rate, parity, or gravidity (P > 0.05); the two groups were comparable. Menopause is medically characterized by the absence of menstruation for a minimum duration of 1 year.
Histologic and immunocytochemical staining
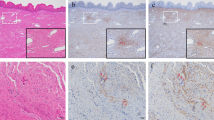
Hematoxylin–eosin (HE) staining demonstrated the features of histology of the intact vagina, including four anatomical layers: stratified squamous epithelial, lamina propria, muscularis, and adventitia (as shown in Supplementary Fig. 1). Figure 1 displays representative images for each stain. The localization of the majority of collagen within the lamina propria was showed through HE, Masson’s trichrome, and immunocytochemical staining. Staining revealed presence of discoloration between the muscle bundles in the layer of muscularis, while no collagen immunostaining was identified in the stratified squamous epithelial layer. In relation to the control group, the appearance of collagen fibers in the lamina propria layer were much more discontinuous, loose, and disordered in the POP group, as depicted in Fig. 1.
Light microscope observations of pictures. (a-a1) HE staining in the control group. (b-b1) HE staining in the POP group. (c-c1) Masson trichrome staining in the control group. (d-d1) Masson trichrome staining in the POP group. (e-e1) Expression of IGFBP5 in the control group. (f-f1) Expression of IGFBP5 in the POP group. (g-g1) Expression of collagen I in the control group. (h-h1) Expression of collagen I in the POP group. (i-i1) Expression of collagen III in the control group. (j-j1) Expression of collagen III in the POP group. (k-k1) Expression of MMP2 in the control group. (l-l1) Expression of MMP2 in the POP group. (m-m1) Expression of TIMP1 in the control group. (n-n1) Expression of TIMP1 in the POP group. Magnification, × 200 (a-n) and × 400 (a1-n1); scale bar: 50 μm.
Immunohistochemistry results for proteins are shown in Fig. 2. The POP group showed markedly lower expression of IGFBP5 compared to the control group (0.2949 ± 0.0045 vs. 0.2997 ± 0.0044, P < 0.01). Similarly, the POP group exhibited reduced expression of collagen I (0.3138 ± 0.0062 vs. 0.3181 ± 0.0018, P < 0.01) and collagen III (0.2687 ± 0.0076 vs. 0.2730 ± 0.0048, P < 0.05) compared to the control group. Conversely, MMP2 expression in the POP group significantly exceeded than that in the control group (0.3066 ± 0.0121 vs. 0.2806 ± 0.0093, P < 0.0001). However, TIMP1 expression in the POP group was comparable to that in the control group (0.2327 ± 0.0025 vs. 0.2329 ± 0.0025, P = 0.865).
Western blotting
Western blot analysis showed a obvious decrease expression of IGFBP5 (1.00 ± 0.8160. vs 0.45 ± 0.2333, P < 0.01) in the POP group. The expression levels of collagen I (1.00 ± 1.2045 vs. 0.16 ± 0.1626, P < 0.01), collagen III (1.00 ± 1.5584 vs. 0.18 ± 0.0815, P < 0.05) were markedly decreased in the POP group compared to the control group, as indicated by statistically significant differences. TIMP1 (1.00 ± 1.4435 vs. 0.40 ± 0.4225, P = 0.084) was lower in the POP group. Conversely, western blot analysis revealed a significant increase expression of MMP2 (1.00 ± 0.8619 vs. 3.11 ± 4.1527, P < 0.05) in the POP group. The results of western blot were corresponded to the immunohistochemical analysis (Fig. 3).
(a) Western blot analysis was used to detect IGFBP5, collagen I, collagen III, MMP2 and TIMP1 protein expression levels in the vaginal tissues of patients in the control and POP groups. (b) Western blot relative expression quantitative analysis of protein IGFBP5, (c) collagen I, (d) collagen III, (e) MMP2, and (f) TIMP1. The results represent the mean and standard deviation. *P < 0.05, ***P < 0.001, ns P > 0.05.
RT-qPCR
As observed in Fig. 4, changes in the levels of mRNA of IGFBP5, collagen I, collagen III, MMP2, and TIMP1 in the two groups were similar to the changes in their corresponding levels of protein. The mRNA level of IGFBP5 (1.00 ± 1.0543 vs. 0.43 ± 0.3842, p < 0.05) in the POP group was obviously decreased compared to that in the control group. Expression of collagen I (1 ± 1.0418 vs. 0.44 ± 0.3731, P < 0.05) and collagen III (1.00 ± 0.9924 vs. 0.50 ± 0.4926, P < 0.05) mRNA levels in the POP group were notably reduced. While MMP2 mRNA level was significantly increased (1.00 ± 0.7221 vs. 4.13 ± 3.2018, P < 0.001). The mRNA level of TIMP1, inhibitor of MMP activity, was similar in two groups (1.00 ± 0.7827 vs. 0.67 ± 0.3458, P = 0.093).
Comparison of IGFBP5
To make further efforts to validate the effect of IGFBP5 in the age and the development of POP, we compared the RT-qPCR and western blot analyses of IGFBP5 in patients with different ages and degrees of prolapse. The expression levels are presented in Table 3. IGFBP5 mRNA expression in POP-Q stage III patients was obviously higher than POP-Q stage IV patients (1.00 ± 0.8741 vs. 0.25 ± 0.1576). Western blot analysis indicated that the IGFBP5 expression level in POP-Q stage III was also higher than that in POP-Q stage IV (1.00 ± 0.5891 vs. 0.31 ± 0.2070). Both the mRNA and protein results were statistically significantly different (P < 0.05). IGFBP5 mRNA (1.00 ± 0.8893 vs. 0.44 ± 0.3576) and protein (1.00 ± 0.6044 vs. 0.48 ± 0.2519) expression levels were obviously higher in younger POP patients (< 65 years old) when compared to older POP patients (≥ 65 years old) (P < 0.05). The correlation between age and IGFBP5 expression levels are presented in Fig. 5. The protein (r = − 0.7145, P < 0.0001) and mRNA (r = − 0.386, P < 0.05) levels were negatively correlated with age. The results of multiple regression analysis suggest that both age (β = − 0.643, P < 0.001) and degree (β = − 0.346, P < 0.01) of prolapse had a negative impact on IGFBP5, with age having a greater impact (Table 4).
Discussion
Within our current investigation, we analyzed the different expression levels of IGFBP5 in the lamina propria tissue among two groups. Patients with POP exhibited decreased expression levels of IGFBP5 protein and mRNA in the lamina propria tissue when compared to the control group. Previous reports have examined the expression and biological activities of IGFBP5 in various tissues, however, there has been no research on the expression of IGFBP5 or its involvement in POP. IGFBP5 is overexpressed in systemic sclerosis and idiopathic pulmonary fibrosis lung tissues16. IGFBP5 is downregulated in head and neck squamous cell carcinoma18 and upregulated in breast cancer and colorectal cancer13,19. IGFBP5 can act as a positive or negative regulator of cellular proliferation and apoptosis under different conditions13. A research study found that IGFBP5 promoted the proliferation of cardiac fibroblasts, while caused apoptosis in cardiac myocytes20. In primary human lung fibroblasts, the addition of exogenously recombinant IGFBP5 and the presence of endogenous IGFBP5 both stimulate the expression of the ECM and profibrotic factors9. While in osteosarcoma cells, endogenous and exogenous IGFBP5 exhibits contrary biological effects21. Li et al. noticed that IGFBP5 was observed to hinder breast cancer cells proliferation through an IGF-dependent pathway in vitro22, whereas IGFBP5 facilitates breast cancer cells proliferation in an IGF-independent manner13. Although IGFBP5 has a wide range of biological activities, its role in POP remains uncertain. As far as we know, no previous research has examined the correlation between declined IGFBP5 and the occurrence and progression of POP.
The results of our study also revealed changes in ECM composition-related proteins in POP patients compared patients without POP. Both collagen I and collagen III expression levels were obviously declined in patients with POP. The connective tissue in the lamina propria tissue of anterior vagina approximates the vaginal fornix (apex) was once believed to be a reflective component of the endopelvic fascia23. Type of collagen most plentiful in interstitial connective tissue are collagen I and collagen III. Collagen I is primarily accountable for the mechanical strength of connective tissue, whereas collagen III and elastin are vital in maintaining tissue elasticity24. As collagen I and collagen III are major constituents of ECM in connective tissue25, maintaining an appropriate equilibrium in the synthesis and degradation of collagen are crucial for ECM. The connective tissue support structure of POP is thought to be damaged in strength rather than elasticity7. MMPs are important enzymes for degrading ECM. MMP2 and MMP 9 mainly decrease the accumulation of collagens26, and MMP2 can degrade type I, II, and III collagen proteins27. According to previous reports, TIMP1 expression decreases in POP patients25,28. TIMP1 inhibits various MMPs, including MMP2 and MMP929. Considering the significant changes and important roles of MMP2 and TIMP1 in collagen degradation and ECM metabolism, we assessed the levels of MMP2 and TIMP1 expression (mRNA and protein). Both mRNA and protein expression of MMP2 was obviously upregulated, while TIMP1 levels downregulated among patients with POP and compared to the patients without POP. The differences in TIMP1 were not statistically significant, possibly due to individual differences or insufficient sample size. Duan et al. found the localization of IGFBP5 within ECM in tissues, as well as its ability to bind numerous ECM proteins30. In a cardiac fibroblasts study, it was observed that the introduction of recombinant IGFBP5 led to an increased ERK phosphorylation, cell proliferation, and mRNA expression of collagen III, MMP2, and MMP920. Zeeh et al. found that IGFBP-5 is likely involved in the increased expression of collagen during colitis31. In this study, our data are partially similar to previous data9,20,31. ECM remodeling is associated with multiple factors. Previous researches have shown that IGFBP5 could enhance the production and accumulation of ECM proteins, as well as by promoting the expression of growth factors in lung and skin tissues9,15,32. This study found the IGFBP5 protein is positive associate with collagen I and collagen III proteins. These findings indicate that IGFBP5 may also be related to the metabolism of the ECM by increased MMP2.We speculate that downregulation of IGFBP5 reduced the fibrotic effect of fibroblasts in POP patients. The decreased IGFBP5 could affect the synthesis and degradation of ECM related proteins (e.g., collagen I, collagen III, and MMP2), resulting in changes in protein levels. Our discovery suggest reduced expression of IGFBP5 may have an effect on the development of POP by influencing the collagen composition and metabolism of the ECM. The discovery we made establishes a foundation of IGFBP5 in the ECM of POP.
In POP patients, the younger POP patients who were under 65 years old exhibited higher levels of IGFBP5 expression compared to elder patients who were over 65 years old. Various studies have provided evidence of a decrease in IGFBP5 levels in humans due to aging or certain diseases10,11. POP is an age-associated disease33, the age of POP patients was negatively correlated with IGFBP5 expression in this study. A research proposed that reduction of IGFBP5 during proposed that the reduction of IGFBP5 during consecutive cell passages promotes the process of replicative senescence in fibroblast cells obtained from mouse embryos17. The involvement of IGFBP5 is indispensable the elevation of reactive oxygen species (ROS) and premature senescence triggered by the IL 6/sIL 6R pathway in human fibroblasts34. Therefore, our results on the relationship between age and IGFBP5 indicate that the decreased expression of IGFBP5 with age may contribute to trigger the senescence and apoptosis of POP fibroblasts, resulting in a decline in collagen synthesis and a rise in degradation within the connective tissue of POP. Furthermore, the comparison of IGFBP5 between POP-Q stage III and IV showed that IGFBP5 is lower in severity of prolapse IV. The findings on the influence of age and severity provide additional evidence that IGFBP5 is effective in the advancement of POP. In summary, IGFBP5 may be a vital indicator for early diagnosis and predict the severity of POP.
Previous researches have investigated the decreased TGF-β35 and the potential mechanisms of TGF-β regulating collagen synthesis and degradation in ECM of POP patients36. Both TGF-β and IGFBP5 are profibrotic factors which can increase collagen but their effects are distinct. IGFBP5 enhances fibrosis but limits scar formation to the mesenchymal compartment by preventing excessive disruption of the epithelium, while TGF-β produces uncontrolled fibrotic response both in wound repairing and idiopathic pulmonary fibrosis37. IGFBP5 can interacted with Bat3 to stabilize the Bat3-TβR complex and sustain TGF-β signaling in liver fibrosis38. In summary, it seems that IGFBP5 and TGF-β have a synergistic effect in fibrosis and IGFBP5 has a protective effect in TGF mediated injury of fibrosis. Our results suggested that the decrease of IGFBP5 may relate to the ECM alteration of POP patients. As the possible mechanism of action for IGFBP5 in liver fibrosis38, IGFBP5 may interact with Bat3 to sustain the ECM regulatory effect of TGF-β in POP. Since IGFBP5 plays an important role in the MAPK/ERK pathway in fibrotic diseases such as systemic sclerosis and idiopathic pulmonary fibrosis15, their association with the MAPK/ERK pathway may stimulate ECM production in vaginal tissue of POP. Nevertheless, it is necessary to confirm these potential mechanisms through additional experiments.
There are limitations to this study. Some unsatisfactory results may be limited by the small sample size. We only compared the different expression levels of IGFBP5 and ECM proteins in vaginal wall tissues of patients with POP and without POP but did not investigate how IGFBP5 acts on ECM and participates in the pathogenesis of POP in this study. We also cannot confirm whether the decrease in IGFBP5 causes POP or POP leads to the decrease in IGFBP5. It is important to evaluate the function of IGFBP5 through further in vitro and in vivo experiments.
Overall, the levels of IGFBP5 and proteins related to ECM were significantly reduced in vaginal tissues of POP patients. The expression levels of IGFBP5 showed a negative correlation with both age and the severity of prolapse. The findings of our research indicate that decrease in IGFBP5 expression may play a crucial part in the aging process and the occurrence of POP. IGFBP5 may be a protective factor for POP.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Collins, S. & Lewicky-Gaupp, C. Pelvic organ prolapse. Gastroenterol. Clin. North Am. 51(1), 177–193. https://doi.org/10.1016/j.gtc.2021.10.011 (2022).
Li, Y. et al. Single-cell transcriptome profiling of the vaginal wall in women with severe anterior vaginal prolapse. Nat. Commun. 12(1), 87. https://doi.org/10.1038/s41467-020-20358-y (2021).
Feng, J. et al. ATF3 regulates oxidative stress and extracellular matrix degradation via p38/Nrf2 signaling pathway in pelvic organ prolapse. Tissue Cell 73, 101660. https://doi.org/10.1016/j.tice.2021.101660 (2021).
Qiu, J. et al. Klotho protein reduced the expression of matrix metalloproteinase-1 (MMP-1) and matrix metalloproteinase-3 (MMP-3) in fibroblasts from patients with pelvic organ prolapse (POP) by down-regulating the phosphorylation of ERK1/2. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25, 3815–3824. https://doi.org/10.12659/msm.913623 (2019).
Zeng, C. et al. Correlation between autophagy and collagen deposition in patients with pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 24(3), 213–221. https://doi.org/10.1097/spv.0000000000000455 (2018).
Eckhardt, S. et al. The impact of diabetes mellitus on pelvic organ prolapse recurrence after robotic sacrocolpopexy. Int. Urogynecol. J. 34(8), 1859–1866. https://doi.org/10.1007/s00192-023-05455-y (2023).
Nguyen, X. X., Renaud, L. & Feghali-Bostwick, C. Identification of impacted pathways and transcriptomic markers as potential mediators of pulmonary fibrosis in transgenic mice expressing human IGFBP5. Int. J. Mol. Sci. https://doi.org/10.3390/ijms222212609 (2021).
Li, J., Jiang, Y. & Zhai, X. Circ_0008450 regulates keloid-derived fibroblast proliferation, migration, invasion and apoptosis with increased IGFBP5 through sponging miR-1224-5p. Burns 49(6), 1392–1402. https://doi.org/10.1016/j.burns.2022.12.014 (2023).
Nguyen, X. X. et al. IGFBP-5 promotes fibrosis via increasing its own expression and that of other pro-fibrotic mediators. Front. Endocrinol. 9, 601. https://doi.org/10.3389/fendo.2018.00601 (2018).
Dennis, R. A. et al. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol. Genom. 32(3), 393–400. https://doi.org/10.1152/physiolgenomics.00191.2007 (2008).
Mohan, S., Farley, J. R. & Baylink, D. J. Age-related changes in IGFBP-4 and IGFBP-5 levels in human serum and bone: Implications for bone loss with aging. Prog. Growth Factor Res. 6(2–4), 465–473. https://doi.org/10.1016/0955-2235(95)00027-5 (1995).
Yang, X. et al. Transcriptional profile of human thymus reveals IGFBP5 is correlated with age-related thymic involution. Front Immunol. 15, 1322214. https://doi.org/10.3389/fimmu.2024.1322214 (2024).
Li, X. et al. Expression level of insulin-like growth factor binding protein 5 mRNA is a prognostic factor for breast cancer. Cancer Sci. 98(10), 1592–1596. https://doi.org/10.1111/j.1349-7006.2007.00565.x (2007).
Song, C. et al. IGFBP5 promotes diabetic kidney disease progression by enhancing PFKFB3-mediated endothelial glycolysis. Cell Death Dis. 13(4), 340. https://doi.org/10.1038/s41419-022-04803-y (2022).
Yasuoka, H. et al. NADPH oxidase-mediated induction of reactive oxygen species and extracellular matrix deposition by insulin-like growth factor binding protein-5. Am. J. Physiol. Lung Cell Mol. Physiol. 316(4), L644–L655. https://doi.org/10.1152/ajplung.00106.2018 (2019).
Nguyen, X. X. et al. Phenotypic characterization of transgenic mice expressing human IGFBP-5. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22010335 (2020).
Nojima, I. et al. Downregulation of IGFBP5 contributes to replicative senescence via ERK2 activation in mouse embryonic fibroblasts. Aging 14(7), 2966–2988. https://doi.org/10.18632/aging.203999 (2022).
Hung, P. S. et al. Insulin-like growth factor binding protein-5 (IGFBP-5) suppresses the tumourigenesis of head and neck squamous cell carcinoma. J. Pathol. 214(3), 368–376. https://doi.org/10.1002/path.2280 (2008).
Deng, Y. et al. IGFBP5 is upregulated and associated with poor prognosis in colorectal cancer. Int. J. Gen. Med. 15, 6485–6497. https://doi.org/10.2147/ijgm.s370576 (2022).
Song, S. E. et al. IGFBP5 mediates high glucose-induced cardiac fibroblast activation. J. Mol. Endocrinol. 50(3), 291–303. https://doi.org/10.1530/jme-12-0194 (2013).
Yin, P., Xu, Q. & Duan, C. Paradoxical actions of endogenous and exogenous insulin-like growth factor-binding protein-5 revealed by RNA interference analysis. J. Biol. Chem. 279(31), 32660–32666. https://doi.org/10.1074/jbc.m401378200 (2004).
Butt, A. J. et al. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J. Biol. Chem. 278(32), 29676–29685. https://doi.org/10.1074/jbc.m301965200 (2003).
Delancey, J. O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstetrics Gynecol. 170(6), 1713–1720. https://doi.org/10.1016/s0002-9378(94)70346-9 (1994) (discussion 20–3).
Tian, Z. et al. The difference in extracellular matrix metabolism in women with and without pelvic organ prolapse: A systematic review and meta-analysis. Bjog 131(8), 1029–1041. https://doi.org/10.1111/1471-0528.17768 (2024).
Zhu, Y. P. et al. Evaluation of extracellular matrix protein expression and apoptosis in the uterosacral ligaments of patients with or without pelvic organ prolapse. Int. Urogynecol J. 32(8), 2273–2281. https://doi.org/10.1007/s00192-020-04446-7 (2021).
Shen, J. et al. Distribution and dynamic changes in matrix metalloproteinase (MMP)-2, MMP-9, and collagen in an in stent restenosis process. Eur. J. Vasc. Endovasc. Surg. 61(4), 648–655. https://doi.org/10.1016/j.ejvs.2020.11.035 (2021).
Patterson, M. et al. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 503(2–3), 158–162. https://doi.org/10.1016/s0014-5793(01)02723-5 (2001).
Zhao, Y. et al. Transforming growth factor beta 1 and p44/42 expression in cardinal ligament tissues of patients with pelvic organ prolapse. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 27, 930433. https://doi.org/10.12659/msm.930433 (2021).
Pietrzak, J. et al. Correlation of TIMP1-MMP2/MMP9 gene expression axis changes with treatment efficacy and survival of NSCLC patients. Biomedicines https://doi.org/10.3390/biomedicines11071777 (2023).
Duan, C. & Allard, J. B. Insulin-like growth factor binding protein-5 in physiology and disease. Front. Endocrinol. 11, 100. https://doi.org/10.3389/fendo.2020.00100 (2020).
Zeeh, J. M. et al. Expression of insulin-like growth factor binding proteins and collagen in experimental colitis in rats. Eur. J. Gastroenterol. Hepatol. 13(7), 851–858. https://doi.org/10.1097/00042737-200107000-00014 (2001).
Pilewski, J. M. et al. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am. J. Pathol. 166(2), 399–407. https://doi.org/10.1016/s0002-9440(10)62263-8 (2005).
Brito, L. G. O. et al. Age and/or postmenopausal status as risk factors for pelvic organ prolapse development: Systematic review with meta-analysis. Int. Urogynecol. J. 33(1), 15–29. https://doi.org/10.1007/s00192-021-04953-1 (2022).
Kojima, H. et al. The STAT3-IGFBP5 axis is critical for IL-6/gp130-induced premature senescence in human fibroblasts. Cell Cycle (Georgetown, Tex) 11(4), 730–739. https://doi.org/10.4161/cc.11.4.19172 (2012).
Zhang, L. et al. Molecular mechanism of extracellular matrix disorder in pelvic organ prolapses. Mol. Med. Rep. 22(6), 4611–4618. https://doi.org/10.3892/mmr.2020.11564 (2020).
Akin, M. N. et al. SMAD2, SMAD3 and TGF-β GENE expressions in women suffering from urge urinary incontinence and pelvic organ prolapse. Mol. Biol. Rep. 48(2), 1401–1407. https://doi.org/10.1007/s11033-021-06220-4 (2021).
Sureshbabu, A. et al. Relative roles of TGF-β and IGFBP-5 in idiopathic pulmonary fibrosis. Pulm. Med. 2011, 517687. https://doi.org/10.1155/2011/517687 (2011).
Zhu, Y. et al. The chromatin remodeling protein BRG1 regulates HSC-myofibroblast differentiation and liver fibrosis. Cell Death Dis. 14(12), 826. https://doi.org/10.1038/s41419-023-06351-5 (2023).
Funding
This work was supported by the National Key R&D Program of China [Grant Number 2021YFC2701302], the National Natural Science Foundation of China [Grant Number 82271613], the Project supported by the Natural Science Foundation of Liaoning Province [Grant Number 2022-MS-081], and Project supported by the Natural Science Foundation of Liaoning Province [Grant Number 2022-BS-072].
Author information
Authors and Affiliations
Contributions
Credit authorship contribution statement Conceptualization: [Y.D., Z.X.]; Methodology: [Y.D.], Software: [Y.D.]; Validation: [Y.D.]; Validation: [Y.D.]; Formal analysis and investigation: [Y.D., Y.C., Y.H. and R.G.], Data curation, [Y.D.], Writing—original draft: [Y.D.]; Writing—review and editing: [Z.X.], Funding acquisition: [Z.X., Y.D., R.G.].
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, Y., Chen, Y., He, Y. et al. Expression of insulin-like growth factor binding protein 5 in the vaginal wall tissues of older women with pelvic organ prolapse. Sci Rep 14, 18353 (2024). https://doi.org/10.1038/s41598-024-69098-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69098-9
- Springer Nature Limited