Abstract
The effect of diabetes mellitus (DM) on individual retinal layers remains incompletely understood. We evaluated the intra-retinal layer thickness alterations in 71 DM eyes with no diabetic retinopathy (DR), 90 with mild DR, and 63 with moderate DR without macular edema, using spectral-domain optical coherence tomography (SD-OCT) and the Iowa Reference Algorithm for automated retinal layer segmentation. The average thickness of 10 intra-retinal layers was then corrected for ocular magnification using axial length measurements, and pairwise comparisons were made using multivariable linear regression models adjusted for gender and race. In DM no DR eyes, significant thinning was evident in the ganglion cell layer (GCL; p < 0.001), inner nuclear layer (INL; p = 0.001), and retinal pigment epithelium (RPE; p = 0.014) compared to normal eyes. Additionally, mild DR eyes exhibited a thinner inner plexiform layer (IPL; p = 0.008) than DM no DR eyes. Conversely, moderate DR eyes displayed thickening in the INL, outer nuclear layer, IPL, and retinal nerve fiber layer (all p ≤ 0.002), with notably worse vision. These findings highlight distinctive patterns: early diabetic eyes experience thinning in specific retinal layers, while moderate DR eyes exhibit thickening of certain layers and slightly compromised visual acuity, despite the absence of macular edema. Understanding these structural changes is crucial for comprehending diabetic eye complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Diabetes mellitus (DM) is a growing global pandemic disease and diabetic retinopathy (DR), a microvascular ocular complication, is a leading cause of vision loss in the working-age population1,2. The diabetic population is estimated to grow from 463 million in 2019 to 700 million by 20453. With the growing diabetic prevalence, the number of vision-threatening DR has been estimated to rise to 44.82 million by 20454. Thus, tackling DR changes at its earlier stage is essential to prevent vision loss. DR is clinically classified as non-proliferative and proliferative based on observation of the retinal vasculature changes by ophthalmoscopy or colour fundus photograph5,6,7. These conventional diagnostic methods are relatively insensitive to subtle structural retinal changes occurring in the early stages of DR8. There are ten distinct retinal layers, which are highly organized and consist of photoreceptor cells, retinal ganglion cells (RGCs), bipolar cells, amacrine cells, horizontal cells, glial cells, endothelial cells, pericytes, and retinal pigment epithelial cell9. Cellular interactions are disturbed in DM, causing the loss of almost all retinal cell populations10.
Advanced imaging technology like spectral domain optical coherence tomography (SD-OCT) offers a non-invasive and high-resolution approach to understanding DR's pathophysiology by providing detailed in vivo images of retinal layers11,12. Various studies using SD-OCT have revealed changes in retinal layer thickness in diabetic patients8,13, detecting early neurodegenerative thinning in inner retinal layers even before DR14,15. Another study observed that the outer retinal layers were thicker in DR eyes than in DM no DR16. However, prior studies have certain limitations, including not accounting for the effect of OCT magnification17,18 and limitations in software that combines retinal layers13,16,19,20,21, hindering precise differentiation and measurement of individual layers.
These limitations can be overcome by advanced software segmentation of individual retinal layers. The Iowa Reference Algorithms (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA) contain the OCT Explorer module, which includes OCT segmentation. It is a device-independent tool that is publicly available for segmenting ten retinal layers in volumetric OCT images22,23. When SD-OCT images are coupled with segmentation algorithms, accurate measurements of the thickness of individual retinal layers are obtained12. This automation overcomes manual segmentation limitations, which are time-consuming and have a significant inter-observer variation24. Age-related changes in individual retinal layers have been demonstrated using this method25. Additionally, most studies have evaluated changes in combined DR groups compared to no DR or healthy groups rather than separate DR stages, such as mild and moderate DR12,13,19,20. Our study addresses these limitations by accounting for the effect of OCT magnification using individuals’ axial length values and employing advanced software segmentation to analyze individual retinal layers across a spectrum of no DR to moderate DR in diabetic subjects without DME. Current treatment also focuses on sight-threatening severe nonproliferative and proliferative DR groups. In contrast, no DR and mild and moderate DR groups are being monitored26. Understanding pathological changes in distinct DR stages may allow the development of potential therapies targeting earlier stages of the disease. Hence, the answer to how DM/DR severity differentially affects different retinal layers is required as it is not yet completely understood.
To address these limitations, our study aims to assess changes in individual retinal layers among subjects with diabetes without diabetic macular edema (DME), covering a spectrum from no DR to moderate non-proliferative DR. This range encompasses stages where the standard treatment does not involve active ocular intervention, shedding light on how DM/DR severity impacts distinct retinal layers. Understanding these changes in different DR stages could lead to the development of therapies targeting early disease stages.
Methods
Study participants
We conducted a cross-sectional study. The time frame for the research participants was October 2019–March 2021. The participants were enrolled in the Singapore Imaging Eye Network (SIENA) study27,28, which recruited patients aged 21 and above, either type 1 or type 2 diabetic and normal patients from the Singapore National Eye Center (SNEC). The study's inclusion criteria also included (1) diabetic eyes with no DR, mild DR or moderate DR without any history of intravitreal or retinal laser therapy (2) normal controls defined as patients having no history of DM with no evidence of any other ocular pathology. The study did not include eyes with co-existing eye disorders such as diabetic macular edema, age-related macular degeneration, uveitis, glaucoma, and other vascular occlusive diseases. Exclusion criteria were also extended to participants with significant cataracts or other media opacities that could interfere with imaging quality. Participants with a history of intravitreal therapy, retinal laser, vitrectomies, or medications that might affect the retina were also excluded. They were age-matched by excluding the younger population from the normal cohort. Data for this study included 128 normal, 44 DM no DR, 58 mild DR, and 48 moderate DR patients. Eyes with co-existing eye disorders such as significant media opacity, or DME were excluded from the study. The normal patients were defined as having no history of DM with no evidence of ocular pathology. Ethics approval was obtained from the SingHealth Centralized Institutional Review Board (protocol number R1500/83/2017). Written informed consent was obtained from all participants in adherence to the Declaration of Helsinki.
Ocular examination
Participants were interviewed to obtain demographic data and ocular and medical history. Each participant underwent a standardized eye examination, including measurement of visual acuity using a logarithm of the minimum angle of resolution chart (LogMAR, The Lighthouse, New York, NY), refractive error using autorefraction keratometry (Canon RK-5 Autorefractor Keratometer, Canon, Inc), axial length from IOLMaster 700 (Zeiss, CA, USA) and anterior and posterior segment examination using slit lamp biomicroscopy at SNEC. The spherical equivalent of the refractive error was calculated as the sum of the sphere power with half of the cylinder power. The severity of DR was assessed by a retinal physician using a modified Arlie house classification for DR staging6.
Optical coherence tomography
The participant's characteristics were masked from the single trained technician who performed all scans on the same day with other measurements, using the Cirrus AngioPlex HD-5000 Spectral-Domain OCT (Carl Zeiss Meditec, Inc, Dublin, CA, USA). Each participant received one 6 × 6 mm macula-centered OCT scan (200 A-scans × 200 B-scans). A single trained grader reviewed the quality of OCT scans masked to the participant’s characteristics. Eyes with poor quality images (signal strength index below 6, movement artifacts causing off-centration, breakages, the local weak signal caused by artifacts such as floaters, algorithm failures resulting in segmentation errors), and missing variables were excluded from the analysis29,30,31.
Automated segmentation of individual retinal layers
Automated retinal layer segmentation was performed on the macular OCT images using Iowa Reference Algorithms (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA)32 We segmented ten individual retinal layers: retinal nerve fiber layer (RNFL); ganglion cell layer (GCL); inner plexiform layer (IPL); inner nuclear layer (INL); outer plexiform layer (OPL); outer nuclear layer (ONL); photoreceptor inner/outer segments (IS/OS); outer segment of photoreceptors (OSP); outer segment photoreceptor/retinal pigment epithelium complex (OPR); retinal pigment epithelium (RPE) utilizing the Iowa Reference Algorithm (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA, USA). Average retinal thickness values were obtained on all images for the central subfield and the inner and outer rings of a standard ETDRS grid (Fig. 1). The inner ring is an average of the four parafoveal subfields (IS—inner superior, IN- inner nasal, II- inner inferior, IT—inner temporal), and the outer ring of the four perifoveal subfields (OS- outer superior, ON- outer nasal, OI- outer inferior, OT—outer temporal. Total retinal, i.e., entire macular thickness, was also calculated as the distance from the most anterior hyper-reflective line (corresponding to the inner limiting membrane; ILM) to the posterior of the outermost hyper-reflective line (corresponding to the outer boundary of RPE). We have also combined GCL and IPL layer thickness to get GCIPL thickness measurements.
(A) ETDRS grid and macular B-scan with 10 retinal layers of the right eye. Standard ETDRS grid showing central 1 mm. The inner ring is an average of the four parafoveal subfields (IS inner superior, IN- inner nasal, II- inner inferior, IT—inner temporal), and the outer ring of the four perifoveal subfields (OS- outer superior, ON- outer nasal, OI- outer inferior, OT—outer temporal). (B) A screenshot of 10-layer segmentation of an OCT image, produced by the Iowa Reference Algorithms as indicated by the coloured lines and corresponding retinal layers. Layers 1–10 (top to bottom; as defined by the software): 1. retinal nerve fiber layer (RNFL); 2. ganglion cell layer (GCL); 3. inner plexiform layer (IPL); 4. inner nuclear layer (INL); 5. outer plexiform layer (OPL); 6. outer nuclear layer (ONL); 7. photoreceptor inner/outer segments (IS/OS); 8. outer segment photoreceptor (OSP) 9. outer segment photoreceptor/retinal pigment epithelium complex (OPR); 10. retinal pigment epithelium (RPE).
Adjustment for ocular magnification
All analyses were corrected for ocular magnification using the axial length of each eye33. The Littman and modified Bennett formulas were used to calculate true image size17. Briefly, the relationship between the measured OCT image diameter (\(s\)) and the true diameter of fundus (\({s}_{\text{corrected}}\)) is expressed as \({s}_{\text{corrected}}\) \(=p\times q\times s\) , where \(p\) is the magnification factor of the imaging system camera, and \(q\) is the factor related to the eye (\(q=0.01306\times (axial length - 1.82)\)). For the Cirrus system, the value of the magnification factor (p) is 3.38234. According to the formula, \({s}_{\text{corrected}}\) can be calculated based on scan size (\(s = 1.0 mm\)) area as: \({s}_{corrected}=3.382\times 0.01306\times \left(axial length-1.82\right)\times 1.0\).
Statistical analysis
We did a post-hoc power calculation to evaluate the statistical power of the current existing study (128 normal, 44 DM no DR, 58 mild DR, and 48 moderate DR participants) using the means and standard deviations derived from the current study. For the outer region of the GCL thickness between normal controls and DM no DR (30.37 ± 3.8 µm vs. 28.04 ± 2.8 µm), using an alpha error of 5%, we would have a post-hoc power of 99.1%. For the inner region of the IPL thickness between DM no DR and mild DR (37.57 ± 3.3 µm vs. 35.37 ± 4.0 µm), using an alpha error of 5%, we would have a post-hoc power of 86%. For the inner region of the RNFL thickness between mild DR and moderate DR (25.01 ± 3.2 µm vs. 28.18 ± 5.3 µm), using an alpha error of 5%, we would have a post-hoc power of 95.3% (https://clincalc.com/stats/Power.aspx).35.
Continuous variables, such as age, systolic blood pressure, diastolic blood pressure, visual acuity, axial length, spherical equivalent, signal strength of OCT scan, and mean retinal thickness measurements, were presented as the means ± SD and were compared across the groups using analysis of variance (ANOVA). The chi-square test was used for categorical variables, such as gender and race.
The association between clinical diagnosis (normal, DM no DR, mild DR, moderate DR; independent variable) on visual acuity or individual retinal layers (dependent variable) was assessed by multivariable linear regression models adjusted for gender and race. We previously found a trend of thicker total retinal thickness in men compared to women and in Chinese eyes compared to Malay eyes25. Given the higher prevalence of men and Malays within the moderate DR, we wanted to ensure that the association between the clinical diagnosis and the retinal layers is less likely to be due to pre-existing differences in gender and race composition within the study group.
The strength of the association between the individual retinal layer and macular thickness measurements with visual acuity was performed using Pearson’s r correlation coefficient. To avoid α error accumulation due to multiple testing, we used a conservative Bonferroni correction and considered results statistically significant at the level α = 0.05/3 = 0.02. For all other analyses, a P value < 0.05 was considered statistically significant. All the statistical analysis was performed using IBM SPSS Statistics 29.0.1.0 and StataCorp LLC version 16.1.
Results
Patient’s characteristics
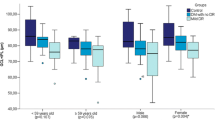
We included 423 eyes from 278 age-matched participants (134 females and 144 males), with a mean age of 59 ± 9. There were 128 (199 eyes) normal, 44 (71 eyes) DM no DR, 58 (90 eyes) mild DR, and 48 (63 eyes) moderate DR participants. Table 1 summarizes the demographic and clinical characteristics of the subjects. A significant gender difference (p = 0.002), race (p < 0.001), and SBP (p = 0.012) was observed across the groups. Notably, the proportion of males (75%), Malays (21%), and higher SBP (145 ± 23 mmHg) were found in the moderate DR group. However, there were no statistically significant differences in axial length (p = 0.134), spherical equivalent (p = 0.083), and OCT signal strength (p = 0.071) across the groups, except for visual acuity (p < 0.001). Individuals with moderate DR had the worst levels of visual acuity (0.22 ± 0.13).
Quantitative retinal layer measurements
Table 2 represents the mean thickness of the individual retinal layers, adjusted for gender and race. All the retinal layers except OSP showed a significant difference across the groups (p trend < 0.05). Specifically, there was a significant impact on the mean retinal thickness in all three regions—central, inner, and outer—in the following layers: RNFL, IPL, INL, OPL, ONL, RPE, combined GCIPL, and the entire macular layer (p trend < 0.05). However, the effects were observed solely in the inner and outer regions of the GCL, the central and inner regions of the IS/OS, and the central area of the OPR layer.
Table 3 shows pairwise comparisons of mean retinal layer thickness between clinical diagnoses (normal, DM no DR, mild DR, moderate DR) using multivariable linear regression analysis, adjusted for gender and race. (A) Normal vs. DM no DR: The GCL (p < 0.001), INL (p = 0.001), and combined GCIPL (p ≤ 0.001) layer showed significant thinning in the outer region in DM no DR eyes when compared to normal eyes. Similarly, the RPE layer also demonstrated decreased thickness in the inner region (p = 0.014). The outer region of macular thickness was significantly thinner (p < 0.001). The RNFL, IPL, OPL, ONL, IS/OS, OSP, and OPR were unaffected in DM with no DR eyes compared to normal eyes. (B) DM no DR vs. Mild DR: The inner regions of GCIPL (p = 0.002) and IPL (p = 0.008) are significantly thinner in mild DR than in DM no DR eyes. (C) Mild DR vs. Moderate DR: The visual acuity in moderate DR eyes was significantly worse than in mild DR groups (p = 0.008). The thickness of the RNFL layer demonstrated significant thickening in inner and outer regions in moderate DR eyes compared to mild DR eyes (p < 0.001). In contrast, the thickness in the central area of GCIPL, mainly the IPL layer, was significantly thicker (p = 0.001). The INL, ONL, and macular area thickness in central, inner, and outer regions increased dramatically in moderate DR eyes compared to mild DR eyes (p ≤ 0.002).
Association of retinal layer thickness with visual acuity
The correlation between the central thickness of ten individual retinal layers with visual acuity is shown in Fig. 2. Worse visual acuity is significantly related to thinner GCL (r = − 0.111, p = 0.022), thinner GCIPL (r = − 0.109, p = 0.025), thicker OSP (r = 0.132, p = 0.007), and thinner OPR (r = − 0.215, p < 0.001). Visual acuity was not significantly correlated to RNFL, IPL, INL, OPL, ONL, IS/OS, RPE, and macular thickness.
Scatter plots show the correlation of GCL, GCIPL, OSP, and OPR with visual acuity (LogMAR). Pearson’s correlation coefficients indicate the strengths of the linear relationship between the variables, and P value < 0.05 is significant. Worse visual acuity is significantly related to thinner ganglion cell layer, thicker outer segment of photoreceptors, thinner outer segment photoreceptor/retinal pigment epithelium complex, and thinner ganglion cell—inner plexiform layer. GCL- Ganglion cell layer; OSP- Outer segment of photoreceptors; OPR- Outer segment photoreceptor/retinal pigment epithelium complex; GCIPL- Ganglion cell-inner plexiform layer.
Discussion
This cross-sectional study employed automatic segmentation Iowa Reference Algorithms software with OCT magnification correction to analyze the thickness of individual retinal layers in diabetic subjects without DME across a spectrum of untreated early to moderate DR stages (no DR to moderate NPDR). This approach addresses limitations of prior research that often relied on combined layer analysis, lacked OCT magnification correction, or focused on treated or later DR stages.
We identified significant differences in most retinal layer thickness (except OSP) among individuals with different stages of DR. Our study found that DM no DR and mild DR resulted in thinning of specific retinal layers instead of moderate DR which led to its thickening, highlighting the importance of differentiating between mild and moderate DR stages, as this distinction can help develop more targeted therapies for the disease at an earlier stage36. Additionally, we observed that in moderate DR cases without macular edema, there was a noticeable decline in visual acuity. This visual acuity decline was significantly associated with thinner GCL, thicker OSP, and thinner OPR. Understanding these correlations provides valuable insights for clinicians in managing visual impairment in diabetic individuals.
Retinal layer thicknesses are decreased in preclinical diabetic retinopathy and mild diabetic retinopathy
Our study revealed significant thinning in GCIPL thickness, with differences observed in the outer region of DM no DR and the inner region of eyes with mild DR. When further analyzed, the thinning in GCIPL thickness in DM no DR was primarily attributed to the GCL. In contrast, mild DR was associated with IPL. This pattern suggests that GCL, composed mainly of cell bodies of RGCs37, is affected earlier in diabetic eyes without apparent vascular signs. In contrast, IPL, which contains numerous synapses connecting RGC dendrites with bipolar and amacrine cells38, is affected later when retinopathy becomes evident. The IPL thinning in mild DR has also been reported in other studies39,40. RGCs are the largest cell bodies among retinal neurons, with higher oxygen consumption than IPL41, thus making them more vulnerable to hypoxia42,43,44,45. Additionally, studies have shown that RGC cell bodies may deteriorate more rapidly in response to injury while retaining their basic dendritic structure46. Since the highest density of ganglion cells is typically found in an elliptical annulus measuring 4.8 × 4.0 mm around the fovea47,48, GCL thinning could be noted earlier in the outer region as found in our study. Although a few studies have separately analyzed the GCL layer using segmentation software, they found no significant thinning in DM no DR eyes. However, these studies had small sample sizes and did not consider OCT magnification correction18,40,49. Our study suggests that analyzing the outer region of the GCL may provide insights into the neurodegenerative aspect of DM before the onset of vascular events.
Retinal layers are increased in moderate diabetic retinopathy
We found that moderate DR eyes experienced thickening in all regions of the entire macula thickness, which is consistent with other studies16,50. On further looking at individual retinal layers, thickening is mainly contributed by INL and ONL in all the regions and thicker GCIPL, particularly IPL, in the foveal area. Moderate DR is characterized by the presence of microaneurysms and dot blot hemorrhages, which may contribute to retinal thickening51. Horii et al. found that 80.3% of microaneurysm resided mainly in INL52. The detection of perfused microaneurysm by SD-OCT positively correlated with retinal thickness53,54. Cytotoxic edema, which is cellular swelling occurring in retinal neurons due to ischemia without apparent vascular leakage, could also contribute to increased retinal thickness55. Additionally, vascular leakage can trigger an inflammatory response, with infiltration of immune cells (microglia) potentially contributing to further thickening56. While some studies57,58,59 found no alterations or thinning in INL or ONL thickness in NPDR eyes without DME, our study with a larger sample size found significant thickening. A seven-year longitudinal study in young individuals with Type 2 diabetes also showed increased combined inner retinal thickness from ILM to OPL in the absence of DME60. The thickening observed in the perifoveal and parafoveal regions of the RNFL contrasts with previous studies reporting thinning in worsening NPDR stages61,62,63,64,65. This thickening could be attributed to cotton wool spots, representing swelling of retinal nerve fiber tissue due to hypoxia common in moderate DR eyes66.
Relationship between retinal layer thickness and visual acuity
Our study has revealed a notable association between the thickness of the OSP and the OPR and visual acuity, with thicker OSP and thinner OPR being linked to worse visual acuity. The outer segments of photoreceptor cells are surrounded by the long apical microvilli of RPE cells, which are responsible for phagocytosing the shed outer segments from photoreceptor cells and returning essential substances to them67,68. This intricate interaction occurs at the apex of RPE-facing photoreceptor cells. Given the high number of photoreceptors in the macula, RPE activity is increased to remove these shed outer segments efficiently69. However, in DM, choriocapillaris nonperfusion70 disrupts this complex RPE-photoreceptor interaction, leading to the accumulation of unengulfed outer segments, possibly explaining the increased thickness of OSP71. Additionally, hypoxia in diabetes interferes with delivering essential nutrients to RPE and photoreceptors72, potentially resulting in a thinner OPR layer. Foveal GCL thinning has been reported in diabetic macular ischemia eyes without macular edema73. These structural alterations have the potential to impact visual function, as supported by electrophysiological studies in DM74,75. Our findings imply that changes in specific retinal layers or structures (such as thinning of GCL and OPR and thickening of OSP) might contribute to decreased visual acuity in cases of moderate DR and may serve as potential biomarkers for the early deterioration of visual acuity, likely indicating the development of DME. While Fig. 2 suggests a relationship between specific retinal layers, such as OSP and OPR, and visual acuity, the strength is weak (OSP: r = 0.132, OPR: r = − 0.215). This may explain why Table 3 did not show significant differences in OSP and OPR thickness between groups, particularly the moderate DR group with poorer visual acuity. Future studies with larger sample sizes are warranted to explore the role of OSP and OPR in visual acuity.
The primary focus of our research was to comprehend how diabetes pathophysiologically affects each distinct retinal layer from the early to moderate stages of DR. By tracking changes in retinal layer thickness, we hope to identify the best time to intervene. Early thinning might signal the start of nerve damage, prompting earlier intervention to prevent further decline, while thickening of retinal layers could imply therapy aimed at halting the advancement of blood-retinal barrier disruption. Therefore, in order to prevent the sight-threatening consequences of DR, it may be beneficial to develop innovative medicines that selectively target a particular retinal layer at an earlier stage.
Strengths and limitations
The strength of our study is in the use of automatic segmentation software with OCT magnification correction to accurately measure individual retinal layer thickness in diabetic eyes in different stages of DR, from no DR up to moderate DR, where currently no treatment is recommended. The potential weakness of the study is that it is a cross-sectional design. Thus, it cannot quantify individual retinal thickness changes over time. Second, we excluded DME eyes, which could interfere with segmenting all retinal layers. Third, treated DR or untreated severe or proliferative DR were not analyzed. Hence, we could not evaluate DR eyes worse than moderate and the effect of treatments such as panretinal photocoagulation and anti-vascular endothelial therapy on the thickness of each retinal layer.
Conclusion
In summary, this study shows a substantial difference in different retinal layer thicknesses across DR severity ranging from no DR to moderate DR. Early diabetic stages such as no DR and mild DR eyes observed thinning in specific retinal layers, whereas eyes with moderate DR experienced thickening. Importantly, even in the absence of DME, visual acuity decline was significantly associated with specific structural changes, including thinner GCL, thicker OSP, and thinner OPR. Understanding these associations is crucial because it allows clinicians to recognize specific structural changes in the eye that might signal the onset or progression of DR. Thus, automatic intraretinal layer segmentation by SD-OCT could play an essential role in DR follow-up and treatment monitoring in identifying and tracking early intraretinal alterations in DR.
Data availability
Data in this study cannot be shared publicly due to regulations of local ethical committees (Sing-Health Centralised Institutional Review Board, R1500/83/2017). Data might be made available to researchers who meet the criteria (to be provided once all data are available) for access to confidential data and upon the Institutional Review Board's approval; requests for access to the dataset can be made to Associate Professor Jacqueline Chua (jacqueline.chua.y.m@seri.com.sg).
References
Ting, D. S. W., Cheung, G. C. M. & Wong, T. Y. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 44, 260–277. https://doi.org/10.1111/ceo.12696 (2016).
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136. https://doi.org/10.1016/S0140-6736(09)62124-3 (2010).
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 157, 107843. https://doi.org/10.1016/j.diabres.2019.107843 (2019).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128, 1580–1591. https://doi.org/10.1016/j.ophtha.2021.04.027 (2021).
Wu, L. Classification of diabetic retinopathy and diabetic macular edema. World J. Diabetes 4, 290. https://doi.org/10.4239/wjd.v4.i6.290 (2013).
Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 98, 786–806 (1991).
Wilkinson, C. P. et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110, 1677–1682. https://doi.org/10.1016/S0161-6420(03)00475-5 (2003).
Jiang, J. et al. Analysis of changes in retinal thickness in type 2 diabetes without diabetic retinopathy. J. Diabetes Res. 2018, 1–7. https://doi.org/10.1155/2018/3082893 (2018).
Miller, D. J., Cascio, M. A. & Rosca, M. G. Diabetic retinopathy: The role of mitochondria in the neural retina and microvascular disease. Antioxidants (Basel) https://doi.org/10.3390/antiox9100905 (2020).
Szabó, K. et al. Histological evaluation of diabetic neurodegeneration in the retina of Zucker Diabetic Fatty (ZDF) Rats. Sci. Rep. 7, 8891. https://doi.org/10.1038/s41598-017-09068-6 (2017).
Chua, J. et al. Optical coherence tomography angiography in diabetes and diabetic retinopathy. J. Clin. Med. 9, 1723. https://doi.org/10.3390/jcm9061723 (2020).
Mohammed, S. et al. Density-based classification in diabetic retinopathy through thickness of retinal layers from optical coherence tomography. Sci. Rep. https://doi.org/10.1038/s41598-020-72813-x (2020).
Ezhilvendhan, K., Shenoy, A., Rajeshkannan, R., Balachandrachari, S. & Sathiyamoorthy, A. Evaluation of macular thickness, retinal nerve fiber layer and ganglion cell layer thickness in patients among type 2 diabetes mellitus using optical coherence tomography. J. Pharm. Bioallied. Sci. 13, S1055-s1061. https://doi.org/10.4103/jpbs.jpbs_165_21 (2021).
Chhablani, J. et al. Neurodegeneration in type 2 diabetes: evidence from spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 56, 6333–6338. https://doi.org/10.1167/iovs.15-17334 (2015).
Tang, Z. et al. Assessment of retinal neurodegeneration with spectral-domain optical coherence tomography: A systematic review and meta-analysis. Eye (Lond) 35, 1317–1325. https://doi.org/10.1038/s41433-020-1020-z (2021).
Dai, W. et al. Macular thickness profile and diabetic retinopathy: The singapore epidemiology of eye diseases study. Br. J. Ophthalmol. 102, 1072–1076. https://doi.org/10.1136/bjophthalmol-2017-310959 (2018).
Higashide, T. et al. Influence of clinical factors and magnification correction on normal thickness profiles of macular retinal layers using optical coherence tomography. PloS One 11, e0147782. https://doi.org/10.1371/journal.pone.0147782 (2016).
Li, S. T., Wang, X. N., Du, X. H. & Wu, Q. Comparison of spectral-domain optical coherence tomography for intra-retinal layers thickness measurements between healthy and diabetic eyes among Chinese adults. PloS One 12, e0177515. https://doi.org/10.1371/journal.pone.0177515 (2017).
Vujosevic, S. & Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 1–8. https://doi.org/10.1155/2013/905058 (2013).
Sánchez-Tocino, H., Alvarez-Vidal, A., Maldonado, M. J., Moreno-Montañés, J. & García-Layana, A. Retinal thickness study with optical coherence tomography in patients with diabetes. Inv. Ophthalmol. Visual Sci. 43, 1588–1594 (2002).
Goebel, W. & Kretzchmar-Gross, T. RETINAL THICKNESS IN DIABETIC RETINOPATHY: A Study Using Optical Coherence Tomography (OCT). RETINA 22 (2002).
Antony, B. et al. Automated 3-D method for the correction of axial artifacts in spectral-domain optical coherence tomography images. Biomed. Opt. Express 2, 2403–2416. https://doi.org/10.1364/boe.2.002403 (2011).
Abràmoff, M. D., Garvin, M. K. & Sonka, M. Retinal imaging and image analysis. IEEE Rev. Biomed. Eng. 3, 169–208. https://doi.org/10.1109/rbme.2010.2084567 (2010).
Chiu, S. J. et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt. Express 18, 19413–19428. https://doi.org/10.1364/oe.18.019413 (2010).
Chua, J. et al. Age-related changes of individual macular retinal layers among Asians. Sci. Rep. 9, 20352. https://doi.org/10.1038/s41598-019-56996-6 (2019).
Wong, T. Y. et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 125, 1608–1622. https://doi.org/10.1016/j.ophtha.2018.04.007 (2018).
Tan, B. et al. Developing a normative database for retinal perfusion using optical coherence tomography angiography. Biomed. Opt. Express 12, 4032–4045. https://doi.org/10.1364/BOE.423469 (2021).
Ahmed, T. S. et al. Ocular microvascular complications in diabetic retinopathy: Insights from machine learning. BMJ Open Diabetes Res. Care https://doi.org/10.1136/bmjdrc-2023-003758 (2024).
Chua, J. et al. Diagnostic ability of individual macular layers by spectral-domain OCT in different stages of glaucoma. Ophthalmol. Glaucoma 3, 314–326. https://doi.org/10.1016/j.ogla.2020.04.003 (2020).
Chua, J. et al. Compensation of retinal nerve fibre layer thickness as assessed using optical coherence tomography based on anatomical confounders. Br. J. Ophthalmol. 104, 282–290. https://doi.org/10.1136/bjophthalmol-2019-314086 (2020).
Chua, J. et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimers Res. Ther. 12, 161. https://doi.org/10.1186/s13195-020-00724-0 (2020).
Zhang, L. et al. Validity of automated choroidal segmentation in SS-OCT and SD-OCT. Invest. Ophthalmol. Vis. Sci. 56, 3202–3211. https://doi.org/10.1167/iovs.14-15669 (2015).
Savini, G., Barboni, P., Parisi, V. & Carbonelli, M. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br. J. Ophthalmol. 96, 57–61. https://doi.org/10.1136/bjo.2010.196782 (2012).
Leung, C.K.-S. et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Inv. Ophthalmol. Vis. Sci. 48, 3178–3183. https://doi.org/10.1167/iovs.06-1315 (2007).
Kane, S. P. ClinCalc LLC: Post-hoc Power Calculator, 2020).
Simó, R., Simó-Servat, O., Bogdanov, P. & Hernández, C. Neurovascular unit: A new target for treating early stages of diabetic retinopathy. Pharmaceutics 13, 1320 (2021).
Sanes, J. R. & Masland, R. H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Ann. Rev. Neurosci. 38, 221–246. https://doi.org/10.1146/annurev-neuro-071714-034120 (2015).
Wright, W. S., Eshaq, R. S., Lee, M., Kaur, G. & Harris, N. R. Retinal physiology and circulation: Effect of diabetes. Compr. Physiol. 10, 933–974. https://doi.org/10.1002/cphy.c190021 (2020).
van Dijk, H. W. et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 50, 3404–3409. https://doi.org/10.1167/iovs.08-3143 (2009).
van Dijk, H. W. et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest. Ophthalmol. Vis. Sci. 53, 2715–2719. https://doi.org/10.1167/iovs.11-8997 (2012).
Yu, D.-Y. et al. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Progress Retinal Eye Res. 36, 217–246. https://doi.org/10.1016/j.preteyeres.2013.07.001 (2013).
Baudry, M. & Bi, X. Calpain-1 and Calpain-2: The Yin and Yang of synaptic plasticity and neurodegeneration. Trends Neurosci. 39, 235–245. https://doi.org/10.1016/j.tins.2016.01.007 (2016).
Zhang, J., Liu, R., Kuang, H. Y., Gao, X. Y. & Liu, H. L. Protective treatments and their target retinal ganglion cells in diabetic retinopathy. Brain Res. Bull. 132, 53–60. https://doi.org/10.1016/j.brainresbull.2017.05.007 (2017).
Qiu, A. W., Bian, Z., Mao, P. A. & Liu, Q. H. IL-17A exacerbates diabetic retinopathy by impairing Müller cell function via Act1 signaling. Exp. Mol. Med. 48, e280. https://doi.org/10.1038/emm.2016.117 (2016).
Cao, Y. et al. Serum response factor protects retinal ganglion cells against high-glucose damage. J. Mol. Neurosci. 59, 232–240. https://doi.org/10.1007/s12031-015-0708-1 (2016).
Kalesnykas, G. et al. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 53, 3847–3857. https://doi.org/10.1167/iovs.12-9712 (2012).
Curcio, C. A. & Allen, K. A. Topography of ganglion cells in human retina. J. Comparative Neurol. 300, 5–25. https://doi.org/10.1002/cne.903000103 (1990).
Zhang, C. et al. Relationship between ganglion cell layer thickness and estimated retinal ganglion cell counts in the glaucomatous macula. Ophthalmology 121, 2371–2379. https://doi.org/10.1016/j.ophtha.2014.06.047 (2014).
van Dijk, H. W. et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 51, 3660–3665. https://doi.org/10.1167/iovs.09-5041 (2010).
Browning, D. J., Fraser, C. M. & Clark, S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology 115, 533-539.e532. https://doi.org/10.1016/j.ophtha.2007.06.042 (2008).
Blair, N. P., Shahidi, M., Lai, W. W. & Zelkha, R. Correlation between microaneurysms and retinal thickness in diabetic macular edema. Retina 28, 1097–1103. https://doi.org/10.1097/IAE.0b013e318177f8ec (2008).
Horii, T. et al. Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am. J. Ophthalmol. 150, 840–848. https://doi.org/10.1016/j.ajo.2010.06.015 (2010).
Wang, H. et al. Characterization of diabetic microaneurysms by simultaneous fluorescein angiography and spectral-domain optical coherence tomography. Am. J. Ophthalmol. 153, 861-867.e861. https://doi.org/10.1016/j.ajo.2011.10.005 (2012).
Wang, H., Chhablani, J., Cheng, L. & Freeman, W. R. The characteristics of diabetic microaneurysms in spectralis optic coherence tomography. Invest. Ophthalmol. Vis. Sci. 52, 1277–1277 (2011).
Lobo, C. L., Bernardes, R. C. & Cunha-Vaz, J. G. Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch. Ophthalmol. 118, 1364–1369. https://doi.org/10.1001/archopht.118.10.1364 (2000).
Zeng, H. Y., Green, W. R. & Tso, M. O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 126, 227–232. https://doi.org/10.1001/archophthalmol.2007.65 (2008).
Kim, S. Y., Jeong, I. W., Yang, Y. S. & Choi, C. W. Changes of the individual retinal layer thickness in non-proliferative diabetic retinopathy in type 2 diabetes. Jkos 58, 1376–1387. https://doi.org/10.3341/jkos.2017.58.12.1376 (2017).
Wanek, J. et al. Alterations in retinal layer thickness and reflectance at different stages of diabetic retinopathy by en face optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 57, 341–347. https://doi.org/10.1167/iovs.15-18715 (2016).
Midena, E., Martini, F., Rediu, A., Casciano, M. & Vujosevic, S. Inner versus outer retinal layers thickness changes in diabetic retinopathy: Spectral domain-OCT automatic detection. Invest. Ophthalmol. Vis. Sci. 51, 4670–4670 (2010).
Mititelu, M. et al. Retinal thickness and morphology changes on OCT in Youth with type 2 diabetes: Findings from the TODAY study. Ophthalmol. Sci. 2, 100191. https://doi.org/10.1016/j.xops.2022.100191 (2022).
Vujosevic, S. & Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 905058. https://doi.org/10.1155/2013/905058 (2013).
Oshitari, T., Hanawa, K. & Adachi-Usami, E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye 23, 884–889. https://doi.org/10.1038/eye.2008.119 (2009).
Nadri, G. et al. Disorganization of retinal inner layers correlates with ellipsoid zone disruption and retinal nerve fiber layer thinning in diabetic retinopathy. J. Diabetes Compl. 33, 550–553. https://doi.org/10.1016/j.jdiacomp.2019.05.006 (2019).
Ulhaq, Z. S. et al. The retinal nerve fiber layer thickness is useful for the assessment of diabetic retinopathy severity. Diabetes Epidemiol. Manag. 7, 100075. https://doi.org/10.1016/j.deman.2022.100075 (2022).
Dhasmana, R., Sah, S. & Gupta, N. Study of retinal nerve fibre layer thickness in patients with diabetes mellitus using fourier domain optical coherence tomography. J. Clin. Diag. Res. JCDR 10, Nc05-09. https://doi.org/10.7860/jcdr/2016/19097.8107 (2016).
McLeod, D., Marshall, J., Kohner, E. M. & Bird, A. C. The role of axoplasmic transport in the pathogenesis of retinal cotton-wool spots. Br. J. Ophthalmol. 61, 177–191. https://doi.org/10.1136/bjo.61.3.177 (1977).
Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881. https://doi.org/10.1152/physrev.00021.2004 (2005).
Kevany, B. M. & Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 25, 8–15. https://doi.org/10.1152/physiol.00038.2009 (2010).
Teirstein, P. S., Goldman, A. I. & O’Brien, P. J. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest. Ophthalmol. Vis. Sci. 19, 1268–1273 (1980).
Bingyao, T. et al. Combining retinal and choroidal microvascular metrics improves discriminative power for diabetic retinopathy. Br. J. Ophthalmol. 107, 993. https://doi.org/10.1136/bjophthalmol-2021-319739 (2023).
Nesper, P. L. et al. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. https://doi.org/10.1167/iovs.17-21787 (2017).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168. https://doi.org/10.1016/j.preteyeres.2009.12.002 (2010).
Byeon, S. H., Chu, Y. K., Lee, H., Lee, S. Y. & Kwon, O. W. Foveal ganglion cell layer damage in ischemic diabetic maculopathy: Correlation of optical coherence tomographic and anatomic changes. Ophthalmology 116, 1949-1959.e1948. https://doi.org/10.1016/j.ophtha.2009.06.066 (2009).
Holopigian, K., Greenstein, V. C., Seiple, W., Hood, D. C. & Carr, R. E. Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 38, 2355–2365 (1997).
Klemp, K. et al. The multifocal ERG in diabetic patients without retinopathy during euglycemic clamping. Invest. Ophthalmol. Vis. Sci. 46, 2620–2626. https://doi.org/10.1167/iovs.04-1254 (2005).
Acknowledgements
This work was funded by grants from the National Medical Research Council (CG/C010A/2017_SERI; OFLCG/004c/2018-00; MOH-000249-00; MOH-000647-00; MOH-001001-00; MOH-001015-00; MOH-000500-00; MOH-000707-00; MOH-001072-06; MOH-001286-00), National Research Foundation Singapore (NRF2019-THE002-0006 and NRF-CRP24-2020-0001), A*STAR (A20H4b0141), the Singapore Eye Research Institute & Nanyang Technological University (SERI-NTU Advanced Ocular Engineering (STANCE) Program), and the SERI-Lee Foundation (LF1019-1) Singapore. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
J.C. conceived and designed the study. All authors collected, analyzed, and interpreted the data. J.S. and J.C. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shah, J., Tan, B., Wong, D. et al. Evaluation of thickness of individual macular retinal layers in diabetic eyes from optical coherence tomography. Sci Rep 14, 17909 (2024). https://doi.org/10.1038/s41598-024-68552-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68552-y
- Springer Nature Limited