Abstract
We aimed to assess the cumulative incidences of cancer-specific mortality (CSM) in non-metastatic patients with non‑muscle invasive urothelial bladder cancer (NMIUBC) and establish competing risk nomograms to predict CSM. Patient data was sourced from the Surveillance, Epidemiology, and End Results database, as well as the electronic medical record system in our institution to form the external validation cohort. Sub-distribution proportional hazards model was utilized to determine independent risk factors influencing CSM in non-metastatic NMIUBC patients. Competitive risk nomograms were constructed to predict 3-year, 5-year, and 8-year cancer-specific survival (CSS) in all patients group, TURBT group and cystectomy group, respectively. The discrimination and accuracy of the model were validated through the concordance index (C-index), the area under the receiver operating characteristic curve (AUC), and calibration curves. Decision curve analysis (DCA) and a risk stratification system was employed to evaluate the clinical utility of the model. Race, age, marital status, surgery in other sites, tumor size, histological type, histological grade, T stage and N stage were identified as independent risk factors to predict CSS in all patients group. The C-index for 3-year CSS was 0.771, 0.770 and 0.846 in the training, testing and external validation sets, respectively. The ROC curves showed well discrimination and the calibration plots were well fitted and consistent. Moreover, DCA demonstrated well clinical effectiveness. Altogether, the competing risk nomogram displayed excellent discrimination and accuracy for predicting CSS in non-metastatic NMIUBC patients, which can be applied in clinical practice to help tailor treatment plans and make clinical decisions.
Similar content being viewed by others
Introduction
Bladder cancer (BLCA) is the most common malignancy affecting the urinary tract and the ninth most common malignancy in the world1. Urothelial cell carcinoma (UCC), formerly known as transitional cell carcinoma (TCC), is the dominant histologic pattern in the US and Western Europe, where it accounts for over 90% of BLCA patients. In other districts worldwide, such as Egypt, Africa and Middle East, non-urothelial carcinomas are also prevalent, which is partly due to the higher prevalence of schistosomiasis2. UCCs arise along the urinary tract, predominantly in the lower urinary tract (bladder and urethra), while arising in the upper urinary tract (renal pelvis and ureters) is also seen. Urologists distinguish non-muscle-invasive bladder cancer (NMIBC) from muscle-invasive bladder cancer (MIBC) according to the depth of infiltration in the bladder. NMIBC belongs to early-stage cancer without invasion into the detrusor muscle layer, composed of carcinoma in situ (CIS; Tis), papillary non-invasive tumors (Ta), and tumors invading the lamina propria (T1)3. MIBC is more likely to result in lymph node metastasis and distant metastasis4.
Non‑muscle invasive urothelial bladder cancer (NMIUBC) is the most common pathological pattern of NMIBC. The standard treatment plan of NMIBC involves transurethral resection of bladder tumor (TURBT), composed of a minimally invasive surgery to resect all visible tumors within the bladder, without breaching and opening up the bladder wall5. Novel endoscopic imaging techniques for carcinoma detection have been applied in clinical practice6, including photodynamic diagnosis (PDD) and narrow-band imaging (NBI), to avoid missing lesions in bladder. Regrettably, relapse and progression after TURBT are common in NMIBC, necessitating the application of adjuvant therapeutic methods7. Intravesical instillations of BCG therapy after TURBT on the same day can mostly decrease the relapse rate by 39% in patients with a low risk of relapse and progression8. However, the oncological outcomes are still unsatisfactory, especially in high-risk NMIBC patients (any T1, high-grade, CIS, or multiple, recurrent and > 3 cm tumors)6, with approximately 21% of patients undergoing progression to MIBC and 13% dying due to BLCA9. NMIBC is an early-stage cancer with rare metastasis occurring10, while it is rather complicated to manage, since tumor relapse and progression are usually observed in clinical practice. Hence, an in-depth comprehension of risk factors for non-metastatic NMIUBC is necessary to optimize tumor management, and a clinical predictive model is urgently in need to precisely predict the prognosis of non-metastatic NMIUBC patients.
Since most patients with non-metastatic NMIUBC have an extended life span11, some patients may pass away due to other-cause mortality, causing a bias to predict cancer-specific mortality (CSM) for clinicians. Dying of non-metastatic NMIUBC may be unforeseen when patients die of other causes involving cardiopulmonary diseases and accidents. Therefore, traditional cox survival analysis method regards other-cause mortality as censored data and neglect the occurrence of competing risk events, causing an overestimation of CSM12. Applying the competing risk model to survival data including competing events is better suited to predict a more precise prognosis. As a result, based on the Surveillance, Epidemiology, and End Results (SEER) database13, we are committed to establishing a competitive risk nomogram as an individual predictive model to assist doctors quantitatively appraise the survival differences in non-metastatic NMIUBC patients.
Results
Patient characteristics
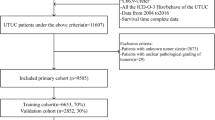
In all patients’ group, we involved 105,288 non-metastatic NMIUBC patients in the analysis, which were randomly divided to the training cohort (n = 73,602) and the validation cohort (n = 31,686). In the training set, the median age was 72.0 years; the death number was 23,201; the CSM number was 6755; and the median follow-up duration was 49.0 months. In the validation set, the median age was 72.0 years; the death number was 9783; the CSM number was 2968; and the median follow-up duration was 51.0 months. In the external validation set, the median age was 72.0 years; the death number was 120; the CSM number was 41; and the median follow-up duration was 53.0 months. The detailed characteristics of patients in the training, validation and external validation cohorts is displayed in Table 1. The TURBT group (n = 97,566) was composed of patients received TURBT therapy, while the cystectomy group (n = 2441) consisted of patients underwent partial or complete cystectomy. The clinical information of patients in the TURBT group and the cystectomy group is listed in Supplementary Tables 1 and 2.
Competing risk regression analysis
In the training cohort of all patients’ group, we conducted the univariate and multivariate competing risk analysis to determine the risk factors of CSM and other-cause mortality in non-metastatic NMIUBC patients (Fig. 1). The univariate competing risk model discovered race, age, marital status, primary tumor site, tumor size, histological type, histological grade, T stage, N stage, surgery type, lymph node surgery, positive lymph node, surgery in other sites and chemotherapy as risk factors of CSM (P < 0.05) (Table 2). Based on the multivariate competing risk analysis, the independent risk factors for non-metastatic NMIUBC patients involved race, age, marital status, tumor size, histological type, histological grade, T stage, N stage and surgery in other sites (P < 0.05) (Table 2). The detailed hazard ratio (HR) and 95% confidence interval (CI) of each factor were listed in Table 2. The 3-year CIF curves of various clinical characteristics in all patients’ group are displayed in Fig. 2. In the training cohort of the TURBT group and the cystectomy group, we also employed the univariate and multivariate competing risk analysis to explore the risk factors of CSM. Based on multivariate competing risk analysis, we found that race, age, marital status, tumor primary site, tumor size, histological type, histological grade, T stage, N stage and chemotherapy were independent risk factors for patients received TURBT (P < 0.05) (Table 3), and race, age, histological grade and N stage were independent risk factors for patients underwent cystectomy (P < 0.05) (Table 3).
Cumulative incidence estimates of cancer-specific mortality (CSM, solid line) and other-casue mortality (OCM, dotted line) according to patient characteristics in non-metastatic NMIUBC patients. (A) age, (B) grade, (C) marital status, (D) tumor size, (E) histology, (F) race, (G) T stage, (H) N stage.
Development and validation of the competitive risk nomogram
In all patients’ group, TURBT group and cystectomy group, respectively, the competing risk nomogram to predict the CSM of non-metastatic NMIUBC patients was established based on the independent risk factors screened by multivariate competing risk analysis in the training cohort (Fig. 3). In all patients’ group, the C-index for 3-year, 5-year, and 8-year CSM in the training cohort was 0.771, 0.746, and 0.720, separately. And the C-index for 3-year, 5-year, and 8-year CSM in the validation cohort was 0.770, 0.746, and 0.723, separately. Meanwhile, the C-index for 3-year, 5-year, and 8-year CSM in external validation cohort was 0.846, 0.864, and 0.798, separately. ROC curve displayed that AUC at 3-year, 5-year, and 8-year in the training cohort were 0.774 (95% CI 0.763–0.785), 0.752 (95% CI 0.742–0.761), and 0.726 (95% CI 0.715–0.736), separately (Fig. 4). And AUC of ROC curve at 3-year, 5-year, and 8-year in the validation cohort was 0.773 (95% CI 0.760–0.787), 0.751 (95% CI 0.739– 0.764), and 0.728 (95% CI 0.715–0.741), separately (Fig. 4). The AUC value of ROC curve at 3-year, 5-year, and 8-year in external validation cohort was 0.851 (95% CI 0.785–0.918), 0.866 (95% CI 0.806– 0.925), and 0.761 (95% CI 0.667–0.855), separately (Fig. 4). In TURBT group and cystectomy group, ROC curves and time-dependent AUC values also showed satisfactory predictive power and well accuracy of the competing risk model (Supplementary Fig. 1). In three groups, calibration curves in training, validation and external validation sets both demonstrated a well consistency with observed outcomes in actual predictions (Fig. 5, Supplementary Fig. 2). Those results showed a well precision and discrimination of the model.
Receiver-operating characteristic curves and time-depending area under the receiver operating characteristic curve for predicting 3-year, 5-year and 8-year cancer-specific mortality in non-metastatic NMIUBC patients. (A,D) Training set. (B,E) Testing set. (C,F) External validation set. The TN stage model is a competing risk model based on T stage and N stage to predict the cancer-specific mortality.
Clinical application
In three groups, we plotted DCA curves to quantitatively evaluate the clinical utility of the model comparing with the TNM staging system. DCA helps clinicians make more informed decisions by calculating the net benefit of using a specific predictive model over no predictive model at all for a range of possible threshold probabilities. As a result, DCA analysis revealed that the greatest net benefit would be derived from using the model to predict CSM for patients at the threshold probability of 0.10 to 0.45 (Fig. 6). Meanwhile, the nomogram was clinically well-behaved in predicting 3-year, 5-year, and 8-year CSM in training, validation and external validation sets, which performed better than the TNM staging system (Fig. 6, Supplementary Fig. 3). Calculating the best cutoff value using Youden index of ROC curve, we constructed a risk stratification system to stratify patients into the high-risk subgroup (risk score ≥ the best cutoff value) and the low-risk subgroup (risk score < the best cutoff value). We conducted CIF curves and Kaplan–Meier curves to verify the risk stratification system, which showed that patients in the high-risk subgroup have significantly higher CSM and other-cause mortality than patients in the low-risk subgroup (p < 0.001, Fine-Gray test and log-rank test), proving the clinical application of our model (Fig. 7, Supplementary Fig. 4).
Decision curve analysis based on the competing risk model for the 3-year, 5-year and 8-year cancer-specific mortality in non-metastatic NMIUBC patients. (A) Training set. (B) Testing set. (C) External validation set. The x-axis represents the threshold probability, and the y-axis represents the net benefit. The TN stage model is a competing risk model based on T stage and N stage to predict the cancer-specific mortality.
Cumulative incidence curve and Kaplan–Meier curves verified that the cancer-specific mortality (CSM) and other-cause mortality (OCM) of patients in the high-risk group were significantly higher than those in the low-risk group in non-metastatic NMIUBC patients. (A,D) Training set. (B,E) Testing set. (C,F) External validation set.
Discussion
BLCA is the ninth most common carcinoma in the world and the most common malignancy affecting urinary system, with over 75% patients initially diagnosed as NMIBC14. Meanwhile, generally 75% of BLCAs are classified as UCC, with histological variants accounting for the rest 25%, stressing a huge disease burden of NMIBC15. Due to the rarity of other histological variants, previous studies were likely to mix different histological variant as one subtype to establish a prognosis model16. Nevertheless, with most of the patients diagnosed as UCC, the comparison between UCC and histological variants is not effective, due to the huge gap in the total number of patients. Otherwise, patients with non-metastatic NMIUBC accounts for the vast majority of BLCA patients, which calls a need of a precise prediction model. Many researchers point out that nomograms perform better than TNM staging system in prognosis prediction for BLCA patients17,18. The drawback of TNM staging system is mostly due to the ignorance of histological disparities and therapeutic regimens, which can be conquered by establishing a competing risk model and risk stratification system especially for non-metastatic NMUIBC patients. In our research, we established a competitive risk nomogram which is more precise and discriminative in prognosis predictions and aligns better with clinical practice.
After univariate and multivariate competitive risk analysis to discover the independent risk factors influencing CSM of non-metastatic NMIUBC patients, we concluded that race, age, marital status, tumor size, histological type, histological grade, T stage, N stage and surgery in other sites are independent risk factors affecting CSM of non-metastatic NMIUBC patients. Race has a huge impact on the prognosis of patients. Researchers discovered that African Americans have lower CSS rates and higher proportions of unfavorable pathology than other mankind19, which may be explained by differences in genetic characteristics, molecular markers, and lifestyles between different races. Age is a significant variable influencing the prognosis of patients in many types of cancer. Our results demonstrated that age not only influenced CSM but also other-cause mortality of non-metastatic NMIUBC patients. Advanced age is the strongest risk factor for BLCA patients, which can be explained by long-term exposure to carcinogens and an age-related reduction in the capability to repair DNA20. Sex is not found significant in our research. Men are there or four times more likely to be diagnosed with BLCA than women, traditionally due to their exposures and lifestyle20, but women are reported to have lower CSS and OS rates, which may be the result of delay diagnosis21. Marriage is found to be a great protective factor for most tumor patients22, because marriage affords patients much financial assistance and psychological console, which is consistent with our result.
In TURBT group, we found that tumors with overlapping lesions have the worst prognosis. And tumors located in bladder base have worse prognosis than that located in urachus or dome, while tumors located in lateral wall of bladder have the best prognosis. It is reported that BLCAs originating in bladder trigone are six times more likely to develop carcinomas of upper urinary tract23, while BLCAs located in trigone and neck of the bladder are more likely to suffer from prostate cancer24. Next, European Association of Urology (EAU) guidelines determined that tumor size is a risk factor for disease progression in BLCA patients25. Researchers also discovered that bigger bladder cancers were significantly correlated with a shorter time duration of tumor relapse26. Our study demonstrated a significant influence of tumor size on CSM in non-metastatic NMIUBC patients. Histological type and various histological variants affect the prognosis of BLCA patients strongly27. Our analysis found that translational cell carcinoma had a worse prognosis than papillary translational cell carcinoma, possibly because translational cell carcinoma is found to be the most common histologic type in metastatic BLCA28. Histological grading is a strong prognostic predictor for progression of NMIBC6. However, different clinical guidelines recommend different pathological reports according to the 1973 or 2004/2016 WHO grading system, involving G1-G3 or low-grade (LG), high-grade (HG) and papillary urothelial neoplasms of low malignant potential (PUNLMP)29, which may bias our analysis.
Nowadays, the TNM staging system is considered the most significant prognostic tool for BLCA patients, providing guidance for the selection of surgical treatment and postoperative supplementary treatment30. We found tumors at T1 stage have the worst prognosis, and much evidence supported that depth and extent of infiltration into the lamina propria (T1 stage) has a great power in prognosis prediction31. To a certain extent, our nomogram has greater capacity in prognosis prediction than TNM staging system. Multimodal therapeutic plan including surgery, chemotherapy, and radiotherapy are the main therapy methods to treat high-risk T1 NMIBC patients32. Chemotherapy is an important treatment option for patients with moderate to high risk NMIBC, especially if BCG therapy fails or does not adapt to BCG therapy. For these patients, chemotherapy can be used as an alternative treatment to reduce the risk of recurrence and improve patient outcomes33. Radiotherapy is considered the main content of trimodal therapy, which includes trans-urethral resection of bladder tumors, chemotherapy, and radiotherapy, which was recommended as an alternate plan for selective patients with MIBC34. But for non-metastatic NMIBC patients, radiotherapy is not recommended. Despite the improvement of radiotherapy, its pelvic toxicity remains severe, which increases the risk of developing secondary primary tumor35. Meanwhile, BLCA patients with distant metastasis usually suffer a worse prognosis with a median OS time seldomly exceeding 3 to 6 months36, which is not comparable with NMIBC patients. Therefore, we did not include NMIUBC patients who received radiotherapy or have distant metastasis into our analysis.
Based on aforementioned risk factors, a competing risk model for CSM in non-metastatic NMIUBC patients was established, which exhibits well discrimination and accuracy through various validation methods. Compared to previous models, our nomogram is more reliable and trustworthy given that we not only focused on CSM but also considered other-cause mortality as competing events, which is of great value in clinical application. Moreover, a risk stratification system was conducted to assist doctors distinguish high-risk patients with poor prognoses, enabling early intervention and early treatment for those patients. Nevertheless, our research has some limitations. Firstly, several prognostic variables are not available in the SEER database, such as imaging modality and its extent, comorbidities, inflammatory markers, genetic factors, and occupational hazards, limiting us from building a more precise model. Secondly, we could not obtain information about progression and recurrence of patients from the SEER database, which is important to predict the prognosis of patients, especially for NMIBC patients with a high possibility to relapse or progress into MIBC. Last but not least, although including a big number of cases, our research is a retrospective study, causing a probability for selection bias and missing data. Future efforts to conduct prospective researches especially multicenter researches to verify the nomogram is suggested.
In conclusion, we utilized the competitive risk analysis to determine the independent risk factors for CSM in non-metastatic NMIUBC patients, and developed nomograms to forecast 3-year, 5-year, and 8-year cancer-specific survival in non-metastatic NMIUBC patients on the basis of the competitive risk model. The nomogram displayed well predictive power in accuracy and discrimination, which can help clinicians in making clinical decision and tailoring individual therapeutic strategies.
Methods
Data sources and extractions
Clinicopathological data of NMIUBC patients was collected from the SEER database (http://seer.cancer.gov/) which involves 18 cancer registries and includes over 28% of US population13. Our SEER database analysis did not require ethical approval and informed consent because patient information from the SEER database is anonymously disclosed. Based on SEER*Stat software 8.4.2, we access the database: Incidence—SEER Research Data, 17 Registries, Nov 2022 Sub (2000–2020). And we collected clinic data of NMIUBC patients in The First Affiliated Hospital of Chongqing Medical University from 2006 to 2023, creating the external validation cohort. The study of external validation was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (protocol code: K2023-338). Informed consent was obtained from all subjects involved in the external validation cohort.
Extracted patient information involves demographic data (age, gender, race, marital status, household location), cancer characteristics (pathological grade, TNM stage, tumor size, tumor primary location, pathology, laterality), therapeutic information (surgery, lymph node surgery, positive lymph node, radiotherapy, chemotherapy) and follow-up information (overall and cancer-specific survival status, survival months). Our selection criteria are (1) NMIBC without metastasis, given that NMIBC patients are seldomly diagnosed as metastasis status37; (2) the first diagnosis was registered as bladder site record (C67.0-C67.9) based on the Third Edition of International Classification of Diseases for Oncology (ICD-O-3). (3) pathology of tumor was translational cell carcinoma (ICD-O-3 8120) and papillary translational cell carcinoma (ICD-O-3 8130). The exclusion criteria are (1) unknown status of distant metastasis or patients with metastasis; (2) unknown surgical type; (3) unknown TNM stage; (4) unknown race; (5) unknown causes of death; (6) survival time less than 1 month; (7) patients without positive diagnostic confirmation; (8) patients received radiotherapy38. Our flowchart for screening patients is displayed in Fig. 1.
Age and tumor size were divided into categorical variables based on the cut-off values obtained by literature review. The age was split into four groups: < 40, 40–59, 60–79, ≥ 80 years. The tumor size was split into five categories: 0–2.0 cm, 2.1–4.0 cm, 4.1–6.0 cm, > 6.0 cm, and unknown. Based on literature review, the primary site of tumors was divided into four groups: "Lateral wall of bladder", "Bladder base", "Urachus/Dome of bladder", "Overlapping lesion of bladder". According to SEER code, the surgery type was categorized into four varieties ("No Surgery", "Local tumor destruction/excision" (TURBT), "Partial cystectomy", "Complete cystectomy"). The survival month was counted from the diagnosis of NMIUBC to the death caused by carcinoma specifically. To establish the competing risk model, we defined survival status as alive (censoring events), mortality caused by NMIUBC (interest events), and mortality caused by other causes (competing events).
Development and evaluation of the competitive risk model
The clinical treatment and management of non-metastatic patients with NMIUBC who undergo TURBT or cystectomy do differ and need to be analyzed separately, so we divided patients into three groups, known as all patients’ group, TURBT group and cystectomy group, to establish three competing risk models respectively. In each group, we randomly allocated patients to a training set (70%) and a validation set (30%). The training set was analyzed to construct the competing risk model and to establish the nomogram. Univariate and multivariate competing risk analyses were employed based on the cumulative incidence function (CIF) and the sub-distribution hazard models, and the differences between subgroups were analyzed by Fine-Gray test39. Based on the sub-distribution proportional hazard model, we considered factors with P value smaller than 0.05 as independent risk factors for CSM. According to the independent risk factors, we established a competitive risk model to forecast the 3-year, 5-year, and 8-year CSS rates of non-metastatic NMIUBC patients.
The validation cohort was utilized to verify the nomogram. The concordance index (C-index) and calibration curves were carried out to evaluate the precision of the competing risk model. The bootstrap method with 1000 times resampling was utilized to internally validate the nomogram. Meanwhile, the model’s discrimination capacity was validated based on the receiver-operating characteristic (ROC) curves and quantified by the area under the ROC curve (AUC) values. Furthermore, we also employed the decision curve analysis (DCA) curves to appraise the clinical utility and survival benefits of the model. Besides, we calculated the risk score of CSS for each patient based on the established nomogram. And we selected the best cutoff value based on Youden index of ROC curve, which was used to build a risk stratification system to divide patients into high-risk and low-risk subgroups. The efficiency of the risk stratification system was appraised by CIF curves and Kaplan–Meier curves.
Statistical analysis
Continued variables were displayed as means and standard deviations, and were compared through Student’s t test. Categorical variables were displayed as absolute number and percentage and were compared through the Pearson or Fisher chi-square test. We analyzed data through R software version 4.2.2 (R Foundation) based on R packages involving cmprsk, rms, pec, prodlim, survival, and riskRegression. Model assessment was conducted with R packages including timeROC, ggDCA. P value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study involving human participants in our institution was reviewed and approved by Ethical committee of the First Affiliated Hospital of Chongqing Medical University (K2023-338). The patients/participants provided their written informed consent to participate in this study.
Data availability
Patient data of SEER database was sourced from https://seer.camcer.gov/. The external validation datasets used and/or analyzed during the current study are available from the corresponding authors (Junhong Liu and Zhikang Yin) upon reasonable request.
Abbreviations
- BLCA:
-
Bladder cancer
- NMIUBC:
-
Non muscle invasive urothelial bladder cancer
- MIBC:
-
Muscle-invasive bladder cancer
- AJCC:
-
American Joint Committee Cancer
- OS:
-
Overall survival
- CSS:
-
Cancer specific survival
- CSM:
-
Cancer specific mortality
- SEER:
-
Surveillance, Epidemiology, and End Results
- HR:
-
Hazard ratio
- CI:
-
Confidential interval
- CIF:
-
Cumulative incidence function
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the receiver operating characteristic curve
- DCA:
-
Decision curves analysis
References
Antoni, S. et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 71(1), 96–108 (2017).
Khaled, H. Schistosomiasis and cancer in Egypt: Review. J. Adv. Res. 4(5), 461–466 (2013).
Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15(1), 25–41 (2015).
Alfred Witjes, J. et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 71(3), 462–475 (2017).
Jones, H. C. & Swinney, J. The treatment of tumours of the bladder by transurethral resection. Br. J. Urol. 34, 215–220 (1962).
Babjuk, M. et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 81(1), 75–94 (2022).
Pawinski, A. et al. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J. Urol. 156(6), 1934–1940 (1996).
Cheung, G., Sahai, A., Billia, M., Dasgupta, P. & Khan, M. S. Recent advances in the diagnosis and treatment of bladder cancer. BMC Med. 11, 13 (2013).
van den Bosch, S. & Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 60(3), 493–500 (2011).
Xu, T. et al. Distant metastasis without regional progression in non-muscle invasive bladder cancer: Case report and pooled analysis of literature. World J. Surg. Oncol. 20(1), 226 (2022).
Cambier, S. et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. Eur. Urol. 69(1), 60–69 (2016).
Austin, P. C., Lee, D. S. & Fine, J. P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 133(6), 601–609 (2016).
Cronin, K. A., Ries, L. A. & Edwards, B. K. The surveillance, epidemiology, and end results (SEER) program of the national cancer institute. Cancer 120(Suppl 23), 3755–3757 (2014).
Wong, M. C. S. et al. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 8(1), 1129 (2018).
Lobo, N. et al. What is the significance of variant histology in urothelial carcinoma?. Eur. Urol. Focus 6(4), 653–663 (2020).
Yang, S. et al. Web-based nomograms for overall survival and cancer-specific survival of bladder cancer patients with bone metastasis: A retrospective cohort study from SEER database. J. Clin. Med. 12(2), 726 (2023).
Zhanghuang, C. et al. Surveillance of prognostic risk factors in patients with SCCB using artificial intelligence: A retrospective study. Sci. Rep. 13(1), 8727 (2023).
Hu, B., Chen, R., Chen, G., Zheng, P. & Fu, B. Prognostic nomogram for estimating survival in patients with resected muscle-invasive bladder cancer receiving chemotherapy. Front Surg. 10, 1121184 (2023).
Gild, P. et al. Racial disparity in quality of care and overall survival among black vs. white patients with muscle-invasive bladder cancer treated with radical cystectomy: A national cancer database analysis. Urol. Oncol. 36(10), 469.e1-469.e11 (2018).
Shariat, S. F., Milowsky, M. & Droller, M. J. Bladder cancer in the elderly. Urol. Oncol. 27(6), 653–667 (2009).
Dobruch, J. et al. Gender and bladder cancer: A collaborative review of etiology, biology, and outcomes. Eur. Urol. 69(2), 300–310 (2016).
Chen, Z. H. et al. Assessment of modifiable factors for the association of marital status with cancer-specific survival. JAMA Netw. Open 4(5), e2111813 (2021).
Palou, J. et al. Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J. Urol. 174(3), 859–861 (2005) (discussion 861).
Pettus, J. A. et al. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. Eur. Urol. 53(2), 370–375 (2008).
Sylvester, R. J. et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 79(4), 480–488 (2021).
Lee, A., Lee, H. J., Huang, H. H., Ho, H. & Chen, K. Low-risk non-muscle-invasive bladder cancer: Further prognostic stratification into the “very-low-risk” group based on tumor size. Int. J. Urol. 26(4), 481–486 (2019).
Wucherpfennig, S. et al. Evaluation of therapeutic targets in histological subtypes of bladder cancer. Int. J. Mol. Sci. 22(21), 11547 (2021).
Shinagare, A. B. et al. Metastatic pattern of bladder cancer: Correlation with the characteristics of the primary tumor. AJR Am. J. Roentgenol. 196(1), 117–122 (2011).
Rubio-Briones, J., Algaba, F., Gallardo, E., Marcos-Rodríguez, J. A. & Climent, M. Recent Advances in the Management of Patients with Non-Muscle-Invasive Bladder Cancer Using a Multidisciplinary Approach: Practical Recommendations from the Spanish Oncology Genitourinary (SOGUG) Working Group. Cancers (Basel) 13(19), 4762 (2021).
Cheng, L., Montironi, R., Davidson, D. D. & Lopez-Beltran, A. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod. Pathol. 22(Suppl 2), S70-95 (2009).
van Rhijn, B. W. et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur. Urol. 61(2), 378–384 (2012).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196(4), 1021–1029 (2016).
Holzbeierlein, J. M. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J. Urol. 211(4), 533–538 (2024).
Arcangeli, G., Strigari, L. & Arcangeli, S. Radical cystectomy versus organ-sparing trimodality treatment in muscle-invasive bladder cancer: A systematic review of clinical trials. Crit. Rev. Oncol. Hematol. 95(3), 387–396 (2015).
Guan, X. et al. Association of radiotherapy for rectal cancer and second gynecological malignant neoplasms. JAMA Netw. Open 4(1), e2031661 (2021).
Wang, P. et al. The role of surgery on the primary tumor site in bladder cancer with distant metastasis: Significance of histology type and metastatic pattern. Cancer Med. 9(24), 9293–9302 (2020).
Ripoll, J. et al. Cancer-specific survival by stage of bladder cancer and factors collected by Mallorca Cancer Registry associated to survival. BMC Cancer 21(1), 676 (2021).
Ślusarczyk, A., Zapała, P., Zapała, Ł, Borkowski, T. & Radziszewski, P. Cancer-specific survival of patients with non-muscle-invasive bladder cancer: A population-based analysis. Ann. Surg. Oncol. 30(12), 7892–7902 (2023).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94(446), 496–509 (1999).
Acknowledgements
We are grateful to the Surveillance, Epidemiology, and End Results (SEER) database for providing data. This work was supported by Special Key Project of Chongqing Technology Innovation and Application Development (No. cstc2019jscx-msxmX0142).
Author information
Authors and Affiliations
Contributions
S.L. and J.W.: Collection and assembly of data, Writing original manuscript, Visualization, Validation. S.L., Z.Z., Y.W. and Z.L.: Data analysis and interpretation, Data curation, methodology. J.L. and Z.Y.: Conception and design, Administration support, Supervision, Manuscript edition, Funding acquisition. All authors contributed to this article and approved the submitted version.
This manuscript has no published elsewhere previously in form of abstract or full text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Wang, J., Zhang, Z. et al. Development and validation of competing risk nomograms for predicting cancer‑specific mortality in non-metastatic patients with non‑muscle invasive urothelial bladder cancer. Sci Rep 14, 17641 (2024). https://doi.org/10.1038/s41598-024-68474-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68474-9
- Springer Nature Limited