Abstract
Objective
To establish a prognostic nomogram among UTUC patients who received chemotherapy.
Methods
1195 UTUC patients who received chemotherapy were extracted from the Surveillance, Epidemiology, and End Results (SEER) database for the period between 2004 and 2015. Patients were randomly divided into a training and a validation set. Nomogram was constructed to predict 1-, 3-, and 5-year overall survival (OS) in those patients. Receiver-operating characteristic curves (ROCs), calibration plots, and Decision curve analysis (DCA) were applied to assess and compare the discrimination, accuracy, and practicability of the nomogram with 8th American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system.
Results
Six clinical parameters were identified as independent prognostic factors for UTUC patients’ OS, including age, marital status, TNM stage, and surgical methods of the primary site. The ROC curves showed a satisfactory discrimination capacity of the nomogram, with 1-, 3-, and 5-year area under curve (AUC) values of 0.789, 0.772, and 0.763 in the training set and 0.772, 0.822, and 0.814 in the validation set, respectively. Calibration curves indicated a good agreement between actual observation and nomogram prediction. ROC and DCA curves showed our nomograms exhibited larger benefits than the 8th AJCC-TNM staging system.
Conclusions
A prognostic nomogram was established and validated to present individual predictions of OS among chemotherapeutic UTUC patients. This nomogram may assist clinicians in accurate survival prognostication, treatment decision-making, and design of future clinical trials.
Similar content being viewed by others
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy, including cancer of the renal pelvis and ureter, which accounts for 5% to 10% of all urothelial tumors [1,2,3]. UTUC is an aggressive tumor with a peak incidence in individuals aged 70–90 years characterized by aggressive growth and variant histology [4]. The high invasiveness of UTUC leads to a poorer prognosis. A study based on the SEER database showed that the 5-year cancer-specific survival (CSS) of UTUC patients was 77% for T2N0 and 39% for lymph node metastasis [5]. Open radical nephroureterectomy (RNU) with bladder cuff excision is the standard treatment of high-risk non-metastatic UTUC. Another option shown to improve survival of patients is peri-operative platinum-based combination chemotherapy [6]. Though large clinical trials are currently lacking to confirm the role of neoadjuvant chemotherapy, it has been shown to result in lower disease recurrence, mortality rates, and an OS and CSS survival benefit compared with RNU alone through several retrospective reviews [7, 8]. In terms of postoperative adjuvant chemotherapy, some scholars have recently confirmed that gemcitabine-platinum combination chemotherapy after nephroureterectomy significantly improved disease-free survival in patients with locally advanced UTUC [9]. For metastatic UTUC patients, systemic chemotherapy (platinum combined chemotherapy) is effective for the first-line treatment of UTUC [6]. Thus far, several scholars have developed corresponding nomograms for UTUC patients to predict patients’ OS and CSS. However, nomograms for patients who received chemotherapy have been lacking. Therefore, an effective prediction model is needed for the accurate assessment of prognosis for these patients, and provide a benchmark for clinical individual decision-making. In this study, we aimed to construct and validate a nomogram for assessing the OS in UTUC patients treated with chemotherapy based on the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Patient and data selection
This study was performed based on the SEER database established by the Department of Cancer Control and Population Sciences of the National Cancer Institute (NCI) in 1973. The database collects data of patients with cancers from 18 districts in the USA, which include clinicopathology, tumor features, and therapeutic details, etc. The database (incidence-seer research plus data, 17 Registries, Nov 2021 Sub (2000–2019)) was used by our study, which covers approximately 26.5% of the U.S. population (based on the 2010 census) and contains one record for each of 8,721,474 tumors. Inclusion criteria were as follows: (1) histologically diagnosed UTUC between 2004 and 2015; 2. Patients who received chemotherapy. The exclusion criteria included the following: 1. Incomplete demographic statistical information such as age, marital status, sex, or race; incomplete clinicopathology information such as tumor-node-metastasis (TNM) stage and pathological grade; incomplete therapeutic information (the interval between diagnosis and treatment, surgical methods of the primary site, or surgery of the regional lymph node); 2. Missing survival status and follow-up information; 3. Diagnostic information from only autopsy or death certificate records; 4. UTUC was not the first primary malignant neoplasm.
SEER * Stat Software (version 8.4.0.1; https://seer.cancer.gov/data-software/) was used to extract information from the SEER database data. 1257 patients with complete clinical data were enrolled in the study, all of which matched the inclusion and exclusion criteria. 2 patients whose survival time was 0 and 60 patients who underwent Multivisceral resection were excluded from the final nomogram. 1195 patients were included in the final model construction. The TNM staging system was reclassified according to version 8 criteria [10].
Statistical analysis
The primary outcome of the study was OS in UTUC patients who received chemotherapy. OS was defined as the interval between the date of cancer diagnosis and the date of death recorded in the registry. 1195 UTUC patients were randomly assigned to the training and validation sets with a ratio of 7:3. The baseline characteristics between the two groups were analyzed by chi-square test and t-test. The training set was used to develop the original nomogram, while the training set and validation set were both used to draw the receiver operating characteristic (ROC) curve, calibration plots, and decision curve analysis (DCA). Univariate and multivariate Cox proportional hazards regression model were applied to determine independent prognostic factors. Then the nomogram was generated based on the independent prognostic factors calculated by the multivariate Cox model. ROC, AUC, and Harrell’s concordance index (C-index) were used to distinguish the models and compare the prediction probabilities of nomogram and 8th AJCC-TNM staging system in 1-year, 3-year, and 5-year OS. Calibration plots were generated to validate the model by comparing the predicted values and actual observations. DCA was applied to quantify clinical utility and compare prognosis predictive capacity between the nomogram and the 8th AJCC-TNM staging system. All tests were performed using R software (version 4.2.1, http://www.R-project.org). Two-sided P < 0.05 was considered statistically significant.
Results
Patient baseline characteristics
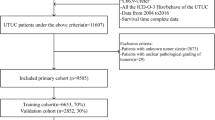
Overall, 5698 UTUC patients were identified from the SEER database, of which 1257 UTUC patients received chemotherapy. 2 patients whose survival time was 0 and 60 patients who underwent Multivisceral resection were excluded. 1195 patients were further assigned to training and validation sets in a 7:3 ratio. Figure 1 shows the screening process. The demographics, clinicopathological characteristics, and therapeutic information of UTUC patients with or without chemotherapy are presented in Table 1. Details of the training and validation sets are provided in Table 2. For UTUC patients who received chemotherapy, the patients were relatively younger (67.2 years vs 72.1 years, P < 0.001) and had a higher grade and TNM stage (P < 0.001) compared with those who did not. The surgery of regional lymph nodes was more common among patients who received chemotherapy (P < 0.001). There were no statistically significant differences in gender (P = 0.076), primary site (P = 0.358), and radiotherapy (P = 1.000). The baseline features between the training and validation sets were well balanced, as shown in Table 2.
Univariate and multivariate analyses
The following variables were included in the univariate cox regression analysis: race, age, gender, marital status, TNM stage, tumor grade, surgical methods of the primary site, radiotherapy, and surgery of regional lymph nodes. Based on the results of the univariate analysis, variables that P < 0.05 were included in the multivariate analysis, including age, marital status, TNM stage, and surgical methods of the primary site. Multivariate Cox regression analysis further confirmed that age, TNM stage, marital status, and surgical methods of the primary site were independent prognostic factors for OS. The results of univariate and multivariate Cox regression analysis are shown in Table 3.
Nomogram construction and validation
Based on multivariate Cox regression analysis, our nomogram was developed to predict OS in UTUC patients who received chemotherapy. In the nomogram depicted in Fig. 2, the score of each variable was calculated by applying a ranking scale drawn by the intersection of the vertical line from each independent factor to the point axis. The 1-, 3-, and 5-year survival probabilities can then be further obtained by summing the scores.
Nomogram predicting 1-, 3-, and 5-year overall survival for UTUC patients treated with chemotherapy. Nomograms were built by using data from the training sets. RNU: Radical nephroureterectomy; PN/PU: Partial nephrectomy/ureterectomy; RN: Radical nephrectomy; LMR/LMD: Local Mass resection/destruction
The C index of our model is significantly higher than that of the TNM staging system (0.7108 VS 0.6723, P < 0.001). As shown in Figs. 3 and 4, our nomogram shows the satisfactory discriminative capacity of OS prediction. The predictive accuracy of our nomogram was superior to that of the AJCC-TNM staging system (P = 0.001 for the training set, P < 0.001 for the validation set). The area under curve (AUC) was 0.789 (1-year), 0.772 (3-year) and 0.763 (5-year) for the training set and 0.772 (1-year), 0.822 (3-year) and 0.814 (5-year) for the validation set, respectively. The calibration plot was used to reflect the agreement between the nomogram prediction and the actual observation of the patients’ OS. The calibration plots indicated good calibration (Fig. 5). Figure 6 shows the comparison of DCA between our nomogram and AJCC-TNM staging system.
Discussion
Nowadays, the AJCC-TNM staging system is a common tool for predicting OS in UTUC patients [6, 11]. However, the TNM staging system only involves the local progression and distant metastasis of the tumor, which leads to a great difference in the final prognosis. A major benefit of a nomogram is that it can provide prognostic values to predict the patient's prognosis more accurately [12]. In our study, data were extracted from the SEER database to establish a prognostic nomogram for UTUC patients who received chemotherapy. To our knowledge, this is the first attempt to establish a predictive model for this subgroup.
Our study showed that patients who received chemotherapy had higher grade and TNM stage than patients who did not. This is consistent with the founding of other scholars [13]. The recent EAU guideline recommends chemotherapy for patients with high-risk non-metastatic UTUC and metastatic UTUC patients [6]. Goldberg, H et al. stated that perioperative chemotherapy had no effect on cancer specific mortality in high-risk non-metastatic patients through SEER database [13]. However, Zhai, T. S. et al. found that the beneficial effect of perioperative chemotherapy on OS was to be evident in pT3/pT4 and pN + patients but to reduce cancer-specific survival for pT1 and OS for pT2 patients [14]. For low-risk UTUC patients, the benefits of perioperative chemotherapy need to be carefully weighed against the risk. Wang, M et al. found that patients who receive chemotherapy was associated with improved overall all survival [15]. All these conclusions need to be interpreted with caution because of the selection bias, residual unmeasured confounding, and lack of timing, protocol, tolerability, and complications of chemotherapy. We still need the large randomized controlled study to access the effects of neoadjuvant chemotherapy and adjuvant chemotherapy, respectively. Clinically, chemotherapy should be individualized for UTUC patients. Except for the grade, stage, or other characteristics of the tumor, the patient's age, health condition, and renal function should also be considered, especially for postoperative patients [16]. Therefore, these patients belong to a special group, and targeted nomograms should be applied to access the prognosis of patients and aid clinicians to make decisions.
Our research indicated that age, TNM stage, marital status, and surgical methods of the primary site were independent prognostic factors for OS of UTUC patients treated with chemotherapy. Our nomogram was developed based on these prognostic factors to predict OS at 1, 3, and 5 years. The nomogram shows good prognostic ability and reliability. ROC and DCA curves showed our nomograms exhibited larger benefits than the 8th AJCC-TNM staging system. The results were consistent in both the training set and the internal validation set. However, Wang, M. suggested the use of AJCC TNM staging may better guide clinical decisions when predicting prognosis in high-grade patients [15]. The full information such as comorbidity are inaccessible from the SEER database, and selection bias could not be avoided in this research. External validation is still needed.
Several nomograms have been established to predict the prognosis of UTUC patients. Wu J et al. established a nomogram based on the SEER database, in which the gender, age, marital status, histology, seer stage, grade, surgery, radiotherapy, and chemotherapy were identified as prognostic factors for patients’ OS [17], Qi F et al. did a similar study, and the model also included radiotherapy [18]. At present, whether radiotherapy can improve the prognosis of UTUC patients is controversial and the combined effect of chemotherapy and radiotherapy remains questionable [6, 19]. In our study, radiotherapy did not improve patients' OS, which needs to be confirmed by further studies. In another study conducted by Li Z et al., three independent factors were identified for patients with invasive UTUC: age, TNM stage, and grade. The nomogram indicated better predictive accuracy than the AJCC-TNM staging system [20], Li C et al. constructed a nomogram by using the competing risk model. They found that LNP and LNR were associated with the CSD of UTUC patients [21]. Previous models mainly focused on the whole UTUC population to construct corresponding models, while our study focused on the special group that received chemotherapy. This is one of the strengths of our study over previous studies.
One further strength lies in the following aspect. Surgical methods were incorporated into our nomogram for predicting the OS of UTUC patients. We built a nomogram based on age, marital status, TNM stage, and surgical methods of the primary site to predict the OS of UTUC patients who received chemotherapy. Surgical methods of the primary site were as follows: local Mass resection/destruction, radical nephroureterectomy, partial nephrectomy/ureterectomy, and radical nephrectomy. Open radical nephroureterectomy with bladder cuff excision is the standard treatment for high-risk non-metastatic UTUC [6]. However, in real clinical practice, the surgical methods need to be combined with the actual situation of the patient. In some cases, such as solitary kidney, severely impaired renal function, or the patient cannot tolerate general anesthesia, Kidney-sparing surgery combined with postoperative chemotherapy is also a kind of choice, so our model was in line with the real clinical condition. The prognostic nomogram that we established displayed a better prognosis prediction capacity compared to the 8th AJCC-TNM staging system. Therefore, for UTUC patients treated with chemotherapy, our nomogram may assist clinicians in accurate survival prognostication, treatment decision-making, and design of future clinical trials. At present, radical nephroureterectomy also has a lot of innovation such as laparoscopic radical nephroureterectomy with only three trocars [22] and these various new techniques can be incorporated into the futural clinical nomogram.
UTUC with histological variants were excluded from our study such as squamous and sarcomatoid variants. Histological variants of urothelial carcinoma are relatively rare, with approximately 25% of UTUC containing variant histology. Variant histology was associated with higher grades and poorer oncological outcomes [23, 24]. The validity of chemotherapy for this subgroup is controversial. A retrospective study demonstrated that the improvement in OS of these patients was not statistically significant [25]. Therefore, these patients were excluded from our study, thereby improving the accuracy of the study.
Our study had certain drawbacks. First, our study was a retrospective research design and selection bias inevitably existed. Second, the SEER database is short of detailed information about specific chemotherapy regimens, comorbidity and renal function. The latter emerges as an important factor influencing whether patients receive chemotherapy and its efficacy. Third, our nomograms should be externally validated for prediction capacity by large cohorts. Moreover, since more that 10% of UTUC patients had concomitant bladder cancer [26], and some patients presented with recurrence in the bladder cancer following treatment [27]. This study did not consider concurrent or heterochronous bladder cancer. Further study is required to address this issue.
Conclusion
At present, no suitable model exists to predict OS in UTUC patients treated with chemotherapy. The prognostic predictive capacity and reliability of our model were acceptable. Our model may provide meaningful reference to assist clinicians in accurate survival prognostication, treatment decision-making, and design of future clinical trials.
Availability of data and materials
The datasets analysed during the current study can be obtained by the SEER*Stat software (https://seer.cancer.gov/). All data are fully available without restriction. The author obtained access to the SEER database (Accession Number: 14467-Nov2021).
Change history
24 June 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12894-023-01231-8
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7–33.
Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107(7):1059–64.
Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, Spiess PE, Lotan Y, Ye D, Fernández MI, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. 2017;35(3):379–87.
Shariat SF, Favaretto RL, Gupta A, Fritsche HM, Matsumoto K, Kassouf W, Walton TJ, Tritschler S, Baba S, Matsushita K, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2011;29(4):481–6.
Rosiello G, Palumbo C, Knipper S, Pecoraro A, Luzzago S, Deuker M, Mistretta FA, Tian Z, Fossati N, Gallina A, et al. Contemporary conditional cancer-specific survival after radical nephroureterectomy in patients with nonmetastatic urothelial carcinoma of upper urinary tract. J Surg Oncol. 2020;121(7):1154–61.
EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam, 2022. ISBN 978-94-92671-16-5
Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. 2021;79(5):635–54.
Meng X, Chao B, Vijay V, Silver H, Margolin EJ, Balar A, Taneja SS, Shah O, Bjurlin MA, Anderson CB, et al. High response rates to neoadjuvant chemotherapy in high-grade upper tract urothelial carcinoma. Urology. 2019;129:146–52.
Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto JWF, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2020;395(10232):1268–77.
Abdel-Rahman O. Revisiting the prognostic heterogeneity of AJCC stage IV carcinomas of the upper urinary tract. Clin Genitourin Cancer. 2018;16(4):e859–65.
Mbeutcha A, Rouprêt M, Kamat AM, Karakiewicz PI, Lawrentschuk N, Novara G, Raman JD, Seitz C, Xylinas E, Shariat SF. Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World J Urol. 2017;35(3):337–53.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70.
Goldberg H, Klaassen Z, Chandrasekar T, Sayyid R, Kulkarni GS, Hamilton RJ, Fleshner NE. Does perioperative chemotherapy improve survival in upper tract urothelial carcinoma? A population based analysis. Oncotarget. 2018;9(27):18797–810.
Zhai TS, Jin L, Feng LM, Zhou Z, Liu X, Liu H, Ma WG, Lu JY, Chen W, Yao XD, et al. Perioperative chemotherapy on survival in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy: a population-based study. Front Oncol. 2020;10:481.
Wang M, Ren X, Wang G, Sun X, Tang S, Zhang B, Xing X, Zhang W, Gao G, Du J, et al. Construction of a survival prediction model for high-and low-grade UTUC after tumor resection based on “SEER database”: a multicenter study. BMC Cancer. 2021;21(1):999.
Kaag MG, O’Malley RL, O’Malley P, Godoy G, Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–7.
Wu J, Chen S, Wu X, Mao W, Wang Y, Xu B, Zheng D, Chen M. Trends of incidence and prognosis of upper tract urothelial carcinoma. Bosn J Basic Med Sci. 2021;21(5):607–19.
Qi F, Wei X, Zheng Y, Sha Y, Lu Y, Li X. Nomograms to predict overall and cancer-specific survival in patients with upper tract urothelial carcinoma: a large population-based study. Transl Androl Urol. 2020;9(3):1177–91.
Czito B, Zietman A, Kaufman D, Skowronski U, Shipley W. Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J Urol. 2004;172(4 Pt 1):1271–5.
Li Z, Li X, Li Y, Liu Y, Du P, Liu Z, Xiao K. A novel nomogram for predicting the survival of patients with invasive upper tract urothelial carcinoma. J Cancer. 2021;12(3):790–8.
Li C, Han D, Huang Q, Xu F, Zheng S, Li X, Zhao F, Feng X, Lyu J. Competing-risks nomogram for predicting cancer-specific death in upper tract urothelial carcinoma: a population-based analysis. BMJ Open. 2021;11(7): e048243.
Al Salhi Y, Fuschi A, Martoccia A, Velotti G, Suraci PP, Scalzo S, Rera OA, Antonioni A, Valenzi FM, Bozzini G, et al. Laparoscopic radical nephroureterectomy with only three trocars: results of a prospective single centre study. Arch Ital Urol Androl. 2022;94(1):7–11.
Zamboni S, Foerster B, Abufaraj M, Seisen T, Roupret M, Colin P, De la Taille A, Di Bona C, Peyronnet B, Bensalah K, et al. Incidence and survival outcomes in patients with upper urinary tract urothelial carcinoma diagnosed with variant histology and treated with nephroureterectomy. BJU Int. 2019;124(5):738–45.
Mori K, Janisch F, Parizi MK, Mostafaei H, Lysenko I, Kimura S, Enikeev DV, Egawa S, Shariat SF. Prognostic value of variant histology in upper tract urothelial carcinoma treated with nephroureterectomy: a systematic review and meta-analysis. J Urol. 2020;203(6):1075–84.
Tully KH, Krimphove Md MJ, Huynh MJ, Marchese M, Kibel AS, Noldus J, Kluth LA, McGregor B, Chang SL, Trinh QD, et al. Differences in survival and impact of adjuvant chemotherapy in patients with variant histology of tumors of the renal pelvis. World J Urol. 2020;38(9):2227–36.
Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2013;31(1):141–5.
Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol. 2012;61(5):1069–70.
Acknowledgements
None.
Funding
This project was supported by Peking University People’s Hospital research and development funds (RDY 2021-21) and Beijing Health Technologies Promotion Program (BHTPP2022082).
Author information
Authors and Affiliations
Contributions
CT: Project development, Data analysis, Manuscript writing; JL: Project development, Data analysis, Manuscript writing; LA: Data analysis; Yang Hong: Data collection; QX: Project development, Manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
SEER is a publicly available database. No ethical approval or informed consent was required to access the data. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Cong Tian and Jun Liu declare conflict of interest that they contributed equally to this work. Remaining all authors (Yang Hong, Lizhe An, and Qingquan Xu) declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, C., Liu, J., An, L. et al. Prognostic nomogram for overall survival in upper urinary tract urothelial carcinoma (UTUC) patients treated with chemotherapy: a SEER-based retrospective cohort study. BMC Urol 23, 2 (2023). https://doi.org/10.1186/s12894-022-01172-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-022-01172-8