Abstract
As climate change continues to modify temperature and rainfall patterns, risks from pests and diseases may vary as shifting temperature and moisture conditions affect the life history, activity, and distribution of invertebrates and diseases. The potential consequences of changing climate on pest management strategies must be understood for control measures to adapt to new environmental conditions. The redlegged earth mite (RLEM; Halotydeus destructor [Tucker]) is a major economic pest that attacks pastures and grain crops across southern Australia and is typically controlled by pesticides. TIMERITE® is a management strategy that relies on estimating the optimal timing (the TIMERITE® date) for effective chemical control of RLEM populations in spring. In this study, we assessed the efficacy of control at the TIMERITE® date from 1990 to 2020 across southern Australia using a simulation approach that incorporates historical climatic data and field experimental data on life history, seasonal abundance, and population level pesticide responses. We demonstrate that moisture and temperature conditions affect the life history of RLEM and that changes in the past three decades have gradually diminished the efficacy of the TIMERITE® strategy. Furthermore, we show that by incorporating improved climatic data into predictions and shifting the timing of control to earlier in the year, control outcomes can be improved and are more stable across changing climates. This research emphasises the importance of accounting for dynamic environmental responses when developing and implementing pest management strategies to ensure their long-term effectiveness. Suggested modifications to estimating the TIMERITE® date will help farmers maintain RLEM control outcomes amidst increasingly variable climatic conditions.
Similar content being viewed by others
Introduction
Understanding the complex interplay between pest population dynamics, life histories, and pest management responses is crucial for developing effective management strategies in the context of changing climate and evolving pest populations1,2. Climate change can significantly impact pest behaviour, distribution, and survival, necessitating the continuous adaptation of pest control approaches3. Furthermore, as pest populations evolve, they may develop resistance to traditional pesticides or exhibit altered life history responses, potentially undermining the efficacy of existing control measures3,4. By studying these interrelated factors, researchers can not only gain a deeper insight into the underlying biological processes governing pest responses to control efforts but also devise more targeted, sustainable, and adaptive strategies that can accommodate the challenges posed by a shifting climate and evolving pest populations5.
The redlegged earth mite (RLEM; Halotydeus destructor [Tucker]) is a common and widespread exotic pest of pastures and most broadacre grain crops in southern Australia (Ridsdill-Smith et al.6). The RLEM has five active life stages: larvae with three pairs of legs, protonymphs, deutonymphs, tritonymphs, and adults. RLEM typically produce three to four generations between autumn and spring. Each generation takes about eight days for eggs to hatch, followed by 20–24 days of development to adulthood; adults then live for 25–56 days7. Populations survive the hot dry summer as diapause eggs retained in the cadavers of adult females in late spring. These diapause eggs do not hatch until cool, wet conditions occur in the following autumn (Fig. 1). Adult mites are approximately 1 mm in length with a velvety black body and eight orange-red coloured legs8 and particularly damaging to emerging plant seedlings. RLEM are commonly controlled using pesticides, however, an over-reliance on prophylactic chemical-based control programs has necessitated more nuanced pest management programs, not least due to increasing pesticide resistance issues9,10. One widely adopted strategy among Australian farmers is TIMERITE®11. This strategy increases the efficacy of chemical control through carefully timed applications in spring that target the mite population immediately prior to the production of diapause eggs, reducing the amount of pesticide required to manage RLEM12.
Schematic of RLEM lifecycle. The synchronised hatching of mites in autumn contributes to high risk to establishing plants. Optimal control occurs in the previous spring before the production of diapause (summer) eggs but not so early that populations are able to recover. The TIMERITE® strategy advises on the suitable timing of control RLEM in spring. Credit: Elia Pirtle, Cesar Australia.
Diapause eggs are largely produced towards the end of spring, but rather than being laid, are retained in the bodies of adult females after they die13. While both diapause (summer) eggs and non-diapause (winter) eggs are impervious to pesticides, diapause eggs are also very resilient to heat and desiccation, enabling mite populations to persist into the next winter growing season14. The TIMERITE® strategy is a pre-emptive strategy (before diapause egg production) that aims to reduce mite abundances in the previous spring rather than during the period of greatest crop risk (egg hatch during crop establishment) (Fig. 1). This strategy utilises a predictive model (the TIMERITE® model), which, for a given location, predicts the date at which 90% of females have produced diapause eggs – control is then applied two weeks prior to this date when mites are only just commencing diapause11. Limiting the number of diapause eggs produced in spring substantially reduces the number of mites that hatch the following autumn and therefore protects emerging plant seedlings, especially in scenarios where pesticides may otherwise be necessary, such as when rotating a field from pasture to canola6,12.
The TIMERITE® model prediction is made based on local daylength and long-term growing season. Long-term growing season is defined as the annual period in which monthly mean temperature is greater than two times the monthly rainfall. The 90% diapause date is given by the estimated equation:
where Y is weeks from 90% diapause for each location, \({x}_{D}\) is daylength in minutes, and \({x}_{G}\) is growing season in months11.
The basis of the TIMERITE® strategy that control should take place two weeks prior to the point of 90% diapause was made on the assumption that this coincides approximately with the commencement of diapause egg production. However, field studies that have tested pesticide applications 4 weeks prior to the 90% diapause date did not detect a loss in efficacy compared with applications 2 weeks prior to 90% diapause, while applications 2 weeks after the 90% diapause date were ineffective15. Other studies have suggested that spraying up to two months prior to 90% diapause could maintain efficacy of control16. This suggests that effective application timings may extend to earlier in the year so long as populations do not undergo another spring generation.
Since the development of the TIMERITE® model more than two decades ago, research has highlighted the important role of temperature and moisture in modulating diapause timing in RLEM17,18. These studies showed that the temperature and moisture conditions experienced by developing mites strongly influences the probability of entering diapause. Temperature and moisture are indirectly captured in the TIMERITE® model through the covariate for long-term growing season. But the model has not updated these values based on changing temperature and moisture regimes in Australia. The findings from Cheng and colleagues (2018, 2019) also suggest a more nuanced treatment of climate may lead to improved predictability of diapause. As climate change continues to alter temperature and moisture regimes, it is important to consider the potential impacts on pest management strategies like TIMERITE®.
Using accumulated research and data on the biology of RLEM, we applied a computer simulation approach to address the following research questions: (1) does the inclusion of temperature and moisture data improve RLEM diapause model estimates? (2) how is the efficacy of the existing TIMERITE® strategy expected to vary with changing climates across the Australian range of RLEM? and (3) can the TIMERITE® model be modified to improve expected control efficacy across variable environments?
Methods
Incorporating climatic variables into diapause prediction
To determine whether the incorporation of temperature and moisture data improves the estimates of diapause status, we fitted multiple logistic regression models to field data with different sets of covariates. The field data on diapause consisted of extensive samples of mite populations across different dates and locations where the diapause status of each RLEM population was determined by dissecting individual mites and then counting and categorising egg diapause status according to egg morphology (Ridsdill-Smith et al.11). Diapause field samples spanned 1990–2002. These were largely collected by Ridsdill-Smith and Pavri and represent 1,354,846 dissected and individually sorted eggs from 61,953 individual females. Collections spanned much of Australia’s grain growing region (Fig. 2); many locations with repeated samples taken both within and across years. Given the large body of work underpinning this research program and relevance to the original TIMERITE® model, it is briefly summarised in Supplementary Information 1. This previously unpublished historical data has been compiled into a single dataset for RLEM diapause and has been uploaded to the CSIRO data portal (https://doi.org/10.25919/xpkr-2f75).
Location of field sites across southern Australia where diapause data for RLEM was collected between 1990 and 2002, compiled into a single dataset and used in this study. Darker points represent repeat sampling at each location. Map created by James Maino with the R programming language.19
Although a small proportion of adult females can produce both winter eggs and diapause eggs together (as well as cryptic diapause eggs that are less easily categorised visually), most female RLEM produce either all winter eggs or all diapause eggs17,18. For example, in the compiled historical dataset, only 0.7% of dissected females contained both diapause eggs and winter eggs. Thus, we modelled the onset of diapause as a random Bernoulli variable with diapause (Y = 1) as the outcome:
The probability of diapause is related to linear covariates via a logit function where X is the matrix of covariates and b is a vector of coefficients.
Covariates included those used in the original TIMERITE® model, i.e., daylength and long-term growing season, as well as additional temperature, moisture, and egg hatch covariates identified by recent research (Table 1). These later covariates include the estimated hatch date in autumn20, which has previously been suggested to influence the onset of diapause13, as well as seasonal temperature and moisture conditions17. We compared three diapause models: model 1 included the covariates for daylength and long-term growing season; model 2 included variables for hatch date, and mean autumn/summer temperature and rainfall (in addition to those of model 1); and model 3 included variables for hatch date, and autumn/summer temperature and rainfall for the present year (in addition to those of model 2). Comparison of model 2 and 3 partitions the effect of mean versus annual covariates.
To account for inter-annual variability in diapause onset, historical daily climatic data was accessed through the Queensland government’s SILO database (https://www.longpaddock.qld.gov.au/silo/ [accessed on 29/04/2024]), which makes gridded Australian climate data available from 1889 to present. Competing models were compared in terms of the change in deviance explained through a likelihood ratio test.
Estimating the efficacy of the TIMERITE® strategy under novel conditions
To estimate the efficacy of the TIMERITE® strategy under earlier control timings and changing climates we required a generic approach that could be used to assess variable control timings, at a variety of locations and annual climatic conditions. Experimental field trials testing the efficacy of the TIMERITE® strategy (against no control) have been previously conducted11,12,15,21 and demonstrate a 70–99% reduction in RLEM numbers in the following autumn, depending on the year and location. However, conducting similar trials for a large number of locations across multiple years would represent a significant undertaking and cost. For this reason, and the availability of existing data on RLEM life history, seasonal abundance, and population level responses to pesticides, we opted for a computer simulation approach. The simulation model was developed as follows.
We took control efficacy to be related to the number of diapause eggs present at the end of the growing season following a pesticide application (the lower the number of diapause eggs, the higher the efficacy)22. Specifically, we took efficacy of control E to be a function of the number of adult females retaining diapause eggs at the end of the growing season with chemical control \({N}_{D}\) and without control \({N}_{D}^{*}\):
The number of diapause eggs at the end of a growing season depends on the cumulative number of adult mites, their mortality rate, and the proportion of the population producing diapause eggs at a given time:
where \({m}_{5}\) is the adult mortality rate, \({p}_{D}\) is the proportion of the population producing diapause eggs, and \({n}_{5}\) is the number of adult mites (the fifth post-embryonic life stage).
The number of adult mites \({n}_{5}\) was taken to be part of a larger system of ordinary differential equations for mite instars 1–5:
where \({n}_{i}\) is the abundance of stage \(i\), \({q}_{i}\) is the transition rate from stage \(i {-} 1\) to \(i\), and \({m}_{i}\) is the mortality rate of stage \(i\) (larva = 1, protonymph = 2, deutonymph = 3, tritonymph = 4, and adult = 5). The number of winter eggs \({n}_{w}\) is used for \({n}_{0}\) (see Eq. 5).
Eggs that are laid and hatch in the current growing season (winter eggs) were expressed as:
where e is the rate of winter egg production, \(N= \sum_{i=1}^{5}{n}_{i}\) and K is the carrying capacity of the field.
The mortality rate of post-embryonic life stages is affected by the concentration of pesticide in the environment, expressed as:
where \({m}_{I,i}\) is the intrinsic mortality rate for stages 1–5 (i.e., pesticides only affect post-embryonic stages) and \({m}_{C}\) is the additional mortality rate associated from any pesticide present in the environment.
Estimating the efficacy of a control application E was thus separated into a diapause status component (\({p}_{D}\)), population dynamics component (\({n}_{i}\)), and a pesticide mortality component (\({m}_{C}\)), which were thoroughly parameterised and validated (see Supplementary Information 2).
Estimating changes in control efficacy with location, changing climates, and alternate control timing models
We explored the effect of changes in climatic conditions on simulated efficacy using the SILO climatic dataset described above across years 1990-2020. We compared the efficacy of the TIMERITE® model against two different versions that relaxed the fixed climate and fixed diapause offset assumptions. These are herein referred to as the Dynamic Climate Fixed Offset (DCFO) model and the Dynamic Climate Dynamic Offset (DCDO) model, respectively. The TIMERITE® model may be thought of as a Fixed Climate Fixed Offset model.
Predictions for the original TIMERITE® model do not change across years and were sourced from an existing dataset of TIMERITE® dates for a broad set of locations across southern Australia (see Ridsdill-Smith et al.11). Although there are several pesticide active ingredients registered to control RLEM in Australia (APVMA, 2024), we focussed our analysis on omethoate, an organophosphorus pesticide, because it has been the principal chemical recommended to use for TIMERITE®12.
For the DCFO model, we estimated the point of 50% diapause using diapause model 2 and simulated a pesticide application at a fixed offset 2 weeks prior. Model 2, which utilises shifting mean climatic conditions, was used to satisfy the practical requirement of knowing the chemical application date well in advance of the management period (i.e., for farm planning purposes). For the DCDO model, we again utilised diapause model 2 but, rather than using a fixed offset, we dynamically selected the chemical application date that maximised simulated efficacy based on Eq. 2. While these modifications allow for dynamic climates, neither addresses potential evolutionary adaptation or phenotypic plasticity that may also drive population level responses to changes in environmental conditions23.
Human and animal rights
No live vertebrates or higher invertebrates were involved in this study.
Results
Impact of climatic variables on diapause status
By integrating supplementary explanatory variables, such as hatch date, cumulative degree days, and cumulative rain for the present year (diapause model 3), estimates of diapause timing were significantly improved compared to when variables from the present year were excluded (diapause model 2; Chi-sq. = 634.9, d.f. = 8, p < 0.001) and when only growing season and daylength were used (diapause model 1; Chi-sq. = 7630.6, d.f. = 13, p < 0.001) (Fig. 3). Diapause model 3 accounted for 28.2% of the residual variance of diapause model 1, while diapause model 2 accounted for 25.8% of the residual variance of diapause model 1 (Table 1). Positive coefficients of the model parameters indicate an acceleration in the timing of diapause onset, and negative coefficients signify a delay (Table 1). Increasing temperature and daylength were estimated to accelerate diapause, while the impact of rainfall on diapause varied depending on the season, exhibiting a strong negative effect in summer and a positive effect in spring. The impact of the long-term growing season was consistently negative across all models, while the effect of hatch date was mostly negative.
Proportion of RLEM populations predicted to be in diapause plotted against observations for a subset of field locations and years (those with 10 or more site visits). At some locations, models 2 and 3 predict multiple lines due to conditions varying across years. Note: locations with less than 10 site visits are excluded from this figure but were included in our analysis. The full dataset used in this study is available at https://doi.org/10.25919/xpkr-2f75.
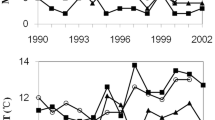
Changes in climatic conditions between 1990 and 2020 were estimated to have reduced the efficacy of the TIMERITE® model by 8.37% (Fig. 3). Across this period, there was a general reduction in growing season length and summer rainfall, but a delay in hatch date and increase in temperature, which contributed to an earlier predicted diapause date (as shown by the direction of the estimated coefficients in Table 1) and reduced TIMERITE® efficacy. In contrast to the TIMERITE® model, which assumed a constant climate across years, the DCFO model adjusted the timing of pesticide applications based on climatic trends, which resulted in a gradual shift toward earlier control dates (Fig. 4). Compared with the TIMERITE® model, the predicted date of the DCFO model was earlier while the pesticide efficacy was higher due to the increased precision of diapause estimation (Table 1). Notably, the DCFO model predicted a higher level of control efficacy by shifting the chemical application dates earlier to account for climatic trends. However, the predicted variation in efficacy of the DCFO model remained high as demonstrated by the large standard deviation across locations (Fig. 4). This large variation in the DCFO model (as well as the TIMERITE® model) indicates that timing pesticide applications at a fixed offset from diapause onset will produce variable results that are dependent on location. The DCDO model however, varied the offset from diapause onset according to local conditions, and predicted high levels of control efficacy with very little variation between locations (Fig. 4). Indeed, the estimated control efficacies are close to the theoretical maximum, which supports the use of diapause model 2 and indicates substitution with diapause model 3 would have little practical impact on pesticide efficacy.
The mean predicted control date and predicted control efficacy of the TIMERITE®, Dynamic Climate Fixed Offset (DCFO), and Dynamic Climate Dynamic Offset (DCDO) models. The points denote the mean across all field locations, the shaded regions denote the standard deviation across locations, and the lines denote the linear best fits.
Using the DCDO model, we estimated the optimal control date for RLEM across a grid of locations encompassing the known distribution of RLEM in southern Australia. Compared with the TIMERITE® model predictions, there was a mean shift of 26.9 days earlier in the season across all locations (Fig. 5). The embedded histogram in Fig. 5 shows the range and distribution of estimated shifts in the optimal date to apply pesticides to achieve maximum control efficacy. These results suggest there is considerable scope to improve control outcomes across most of the RLEM range if the existing TIMERITE® strategy integrates these updated predictions.
Projected shifts in the optimal timing of control as forecasted by the Dynamic Climate Dynamic Offset (DCDO) model, juxtaposed with the predictions from the TIMERITE® model for selected locations spanning the known distribution of RLEM in Australia. In the visualisation, triangle markers indicate a delay, signifying that optimal control is predicted to occur later in the year compared with the TIMERITE® model predictions. Conversely, circle markers indicate an advanced diapause (negative delay), indicating that optimal control is predicted to happen earlier in the year. The colours correspond to the values depicted in the accompanying frequency histogram. Map created by James Maino with the R programming language24.
Discussion
In this study, we explored how pest management strategies may need to adapt to changing climates. We first established that the onset of diapause in field populations of RLEM can be better predicted through the inclusion of moisture and temperature related climatic variables. We then integrated additional data on RLEM biology, population dynamics, and responses to pesticides into a simulation model that was applied to different environmental conditions. This generated estimates of the efficacy of variable pesticide application timings for different locations spanning the Australian range of RLEM using annual climatic conditions from 1990 to 2020. Across this period, the efficacy of the TIMERITE® strategy was predicted to have diminished. Moreover, the control efficacy was predicted to increase substantially when dynamic climatic data and a dynamic (and generally longer) diapause offset were incorporated in the models.
On average, the optimal timing of when to apply pesticides was predicted to be much earlier in the year than the current TIMERITE® model. Warming temperatures, reduced rainfall, and later hatch dates over the past three decades have been observed across much of the known RLEM distribution. These factors are a driving force behind the optimal control dates occurring earlier in the year, and emphasise the importance of adaptive pest management strategies informed by pest ecophysiology. As climatic conditions continue to shift, it will be crucial for pest control measures to remain effective and relevant3. A recent report on the risks and adaptation strategies of Australian agriculture to climate change25 identified altered timing and severity of pest outbreaks as a key threat to farming systems but did not identify suitable methods to address this challenge. We have demonstrated how incorporating climatic processes and life history responses into existing pest management models can ensure adaptable control strategies, helping to safeguard crop yields and economic viability in the face of a changing climate2. Furthermore, the capacity of such approaches to adapt to novel regions and future climates highlights the potential to support farmers in managing emerging pest challenges resulting from further climate change. For instance, the pest status of RLEM may increase in severity in cooler regions where this species is less abundant (e.g., Tasmania and northern New Zealand) as these regions continue to warm. Warmer temperatures may also increase the activity of pesticides against a range of species26, as has already been suggested in RLEM to commonly used pesticides27.
While previous field experiments have demonstrated the high cost of pesticide applications occurring too late15, a key finding of the present work is the opportunity of bringing spray timings earlier in year. This result is largely driven by the long residual toxicity of omethoate, which was estimated to have a mean half-life of 20 days (Supplementary Information 2). Pesticides are ineffective against diapause eggs, but this long window of efficacy allowed the control timing to be extended further into the non-diapause period to maximise the mortality of RLEM populations. Extending the opportunity to achieve effective control to earlier in the year would provide farmers with greater flexibility in control strategies and task scheduling. The authors have developed an online simulation tool to explore these results in more detail for various locations around Australia using updated climatic data (https://cesaraustralia.shinyapps.io/timerite/) and are working directly with industry to update the existing TIMERITE® tool widely used by Australian farmers.
The reduction of spring RLEM populations is currently reliant on long-acting pesticides, which should not be seen as a substitute for integrated pest management practices that utilise the full complement of cultural and biological options for reducing RLEM risk. Pest monitoring and appropriate risk assessments remain a critical component of best practice management. Resistance evolution remains a significant concern in the ongoing management of RLEM, with known cases of resistance increasing while available chemical options diminish9,10,28. Although currently focused on pesticides, the TIMERITE® strategy is more broadly concerned with the identification of a key vulnerability in the life history of RLEM and so could eventually encompass non-chemical control options, such as targeted grazing of pastures by livestock in spring6. The potential benefits of strategic grazing management in spring have been demonstrated in set-stocked pastures, where RLEM populations have decreased from 46,000 to 27/m2 following a four-fold increase in grazing days (total number of sheep multiplied by the number of days grazing)29. Spring grazing reduces habitat suitability for RLEM, thereby suppressing diapause egg production. By incorporating this alternative pest management approach, farmers could achieve a more holistic and sustainable pest control strategy that may further reduce their reliance on pesticides. Integrating targeted grazing would require further research and modelling to determine the optimal timing, intensity, and duration of grazing for maximum suppression of RLEM diapause production. Nonetheless, the foundation established by the comprehensive population and life history analyses conducted in this study can serve as a valuable basis for future endeavours exploring non-chemical pest management options.
Although our study incorporates many processes not included in the original TIMERITE® model, it still omits other details that may affect control outcomes. For instance, recent research on RLEM producing intermediate forms of diapause eggs (cryptic diapause eggs)18 has not been considered in our model, which assumes that diapause status can be determined from morphology alone (see Supplementary Information 3). Additionally, our model does not explicitly consider the small proportion of RLEM diapause eggs that may hatch after two summers, resulting in delayed hatching17. Another knowledge gap surrounds our understanding of the precise physiological triggers of diapause in RLEM11,13,18. The exact life stage at which this trigger occurs remains unclear, as does the potential reversibility of diapause initiation, for example, in response to a return to cooler and wetter conditions in spring. Microclimatic variability and the coarse resolution of climatic data will also limit the precision of model predictions; despite the inclusion of annual climate data, unexplained variation in diapause timing remains, which may be accounted for by microclimatic variation caused by aspect, slope, vegetation composition or biomass within a given field. It is also worth noting that our study did not consider the effects of evolutionary adaptation or phenotypic plasticity in pest responses to changing climates. The field data utilised in this study was opportunistically compiled across a large number of historical studies that would render the investigation of evolutionary adaptation and phenotypic plasticity difficult, if not impossible. However, diapause in other invertebrates has been demonstrated to undergo rapid evolutionary responses to environmental change over a matter of decades30. Additional studies measuring the onset of diapause in geographically distinct RLEM populations in common-garden experiments could help to isolate genetic variability in this trait. It will be important to validate the findings of our work by undertaking field trials aimed at testing the predicted increase in control efficacy against RLEM using the DCDO model.
In addition to the above, there are several research gaps in relation to the cost-benefit of control, including how environmental and climatic conditions influence population size (as opposed to diapause timing) and the conditions under which multiple pesticide applications in spring may be economically warranted (or conversely, if control is not economic). Hard (hot, dry) finishes to the growing season are expected to reduce RLEM population densities compared with soft (cool, wet) conditions, which allow populations to persist longer and egg burdens to increase. While economic thresholds are notoriously difficult to develop for many pests31,32, if population densities surrounding diapause can be accurately related to next autumn populations densities, it may be possible to develop economic thresholds to guide the requirement for spring control. Of course, the disruption to natural biocontrol caused by pesticides33 further complicates such analyses.
In conclusion, our study has demonstrated the importance of considering dynamic environmental responses of pests when developing and implementing management strategies to ensure their long-term effectiveness. Incorporating the underlying biological processes of pest responses to control measures, such as population and life history responses to climatic conditions, can improve pest management outcomes. The findings of the present study help improve the efficacy and resilience of the existing TIMERITE® strategy, which is widely used by farmers to manage RLEM in Australia.
Data availability
The datasets analysed during the current study are available in the CSIRO data portal, https://doi.org/10.25919/xpkr-2f75.
References
Andrew, N. R. & Hill, S. J. Effect of Climate change on insect pest management. In Environmental Pest Management (eds. Coll, M. & Wajnberg, E.) 195–223 (2017). https://doi.org/10.1002/9781119255574.ch9.
Maino, J. L., Kong, J. D., Hoffmann, A. A., Barton, M. G. & Kearney, M. R. Mechanistic models for predicting insect responses to climate change. Curr. Opin. Insect Sci. 17, 81–86 (2016).
Diffenbaugh, N. S., Krupke, C. H., White, M. A. & Alexander, C. E. Global warming presents new challenges for maize pest management. Environ. Res. Lett. 3, 044007 (2008).
Maino, J. L., Umina, P. A. & Hoffmann, A. A. Climate contributes to the evolution of pesticide resistance. Glob. Ecol. Biogeogr. 27, 223–232 (2018).
Hoffmann, A. A., Weeks, A. R., Nash, M. A., Mangano, G. P. & Umina, P. A. The changing status of invertebrate pests and the future of pest management in the Australian grains industry. Aust. J. Exp. Agric. 48, 1481–1493 (2008).
Ridsdill-Smith, T. J. et al. Strategies for control of the redlegged earth mite in Australia. Aust. J. Exp. Agric. 48, 1506–1513 (2008).
Ridsdill-Smith, T. J. Biology and control of Halotydeus destructor (Tucker) (Acarina: Penthaleidae): A review. Exp. Appl. Acarol. 21, 195–224 (1997).
Baker, A. S. A redescription of Halotydeus destructor (Tucker) (Prostigmata: Penthaleidae), with a survey of ontogenetic setal development in the superfamily eupodoidea. Int. J. Acarol. 21, 261–282 (1995).
Umina, P. A., McDonald, G., Maino, J. L., Edwards, O. & Hoffmann, A. A. A. Escalating insecticide resistance in Australian grain pests: Contributing factors, industry trends and management opportunities. Pest Manag. Sci. 75, 1494–1506 (2019).
Arthur, A. L. et al. Learnings from over a decade of increasing pesticide resistance in the redlegged earth mite Halotydeus destructor Tucker.. Pest Manag. Sci. 77, 3013–3024 (2021).
Ridsdill-Smith, T. J., Pavri, C., De Boer, E. & Kriticos, D. Predictions of summer diapause in the redlegged earth mite, Halotydeus destructor (Acari: Penthaleidae) Australia.. J. Insect Physiol. 51, 717–726 (2005).
Ridsdill-Smith, T. J. & Pavri, C. C. Controlling redlegged earth mite, Halotydeus destructor (Acari: Penthaleidae) with a spring spray in legume pastures. Crop Pasture Sci. 66, 938–946 (2015).
Wallace, M. M. H. Diapause in the aestivating egg of Halotydeus destructor (Acari: Eupodidae). Aust. J. Zool. 18, 295–313 (1970).
Wallace, M. M. H. The influence of temperature on post-diapause development and survival in the aestivating eggs of Halotydeus destructor (Acari: Eupipodidae). Aust. J. Zool. 18, 315–329 (1970).
Pavri, C. C. & Ridsdill-Smith, T. (2002) A TIMERITE® issue: Testing importance of spray timing. In Facing the Future: Grassland Society of Victoria 43rd Annual Conference 123–126
Umina, P. A. & Hoffmann, A. A. Diapause and implications for control of Penthaleus species and Halotydeus destructor (Acari: Penthaleidae) in southeastern Australia. Exp. Appl. Acarol. 31, 209–223 (2003).
Cheng, X., Hoffmann, A. A., Maino, J. L. & Umina, P. A. Summer diapause intensity influenced by parental and offspring environmental conditions in the pest mite Halotydeus destructor. J. Insect Physiol. 114, 92–99 (2019).
Cheng, X., Hoffmann, A. A., Maino, J. L. & Umina, P. A. A cryptic diapause strategy in Halotydeus destructor (Tucker) (Trombidiformes: Penthaleidae) induced by multiple cues. Pest Manag. Sci. 74, 2618–2625 (2018).
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. A BibTeX entry for LaTeX users is @Manual{, title = {R: A Language and Environment for Statistical Computing}, author = {{R Core Team}}, organization = {R Foundation for Statistical Computing}, address = {Vienna, Austria}, year = {2021}, url = {https://www.R-project.org/},}
McDonald, G., Umina, P. A., Macfadyen, S., Mangano, P. & Hoffmann, A. A. Predicting the timing of first generation egg hatch for the pest redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Exp. Appl. Acarol. 65, 259–276 (2015).
Gower, J. M. C., Hoffmann, A. A. & Weeks, A. R. Effectiveness of spring spraying targeting diapause egg production for controlling redlegged earth mites and other pests in pasture. Aust. J. Exp. Agric. 48, 1118–1125 (2008).
Ridsdill-Smith, T. J. & Annells, A. J. Seasonal occurrence and abundance of redlegged earth mites Halotydeus destructor (Acari: Penthaleidae) in annual pastures of southwestern Australia. Bull. Entomol. Res. 87, 413–423 (1997).
Anderson, J. T., Inouye, D. W., McKinney, A. M., Colautti, R. I. & Mitchell-Olds, T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. Royal Soc. B: Biol. Sci. 279, 3843–3852 (2012).
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. A BibTeX entry for LaTeX users is @Manual{, title = {R: A Language and Environment for Statistical Computing}, author = {{R Core Team}}, organization = {R Foundation for Statistical Computing}, address = {Vienna, Austria}, year = {2021}, url = {https://www.R-project.org/},}
Sudmeyer, R., Edward, A., Fazakerley, V., Simpkin, L. & Foster, I. Climate change: Impacts and adaptation for agriculture in Western Australia. Bulletin 4870, Department of Agriculture and Food, Western Australia, Perth (2016).
Li, D., Jiang, K., Wang, X. & Liu, D. Insecticide activity under changing environmental conditions: A meta-analysis. J. Pest Sci. 2004, 1–13. https://doi.org/10.1007/s10340-024-01766-1 (2024).
Thia, J. A., Cheng, X., Maino, J., Umina, P. A. & Hoffmann, A. A. Warmer temperatures reduce chemical tolerance in the redlegged earth mite (Halotydeus destructor), an invasive winter-active pest. Pest Manag. Sci. 78, 3071–3079 (2022).
Maino, J. L., Binns, M. & Umina, P. A. No longer a west-side story - Pesticide resistance discovered in the eastern range of a major Australian crop pest, Halotydeus destructor (Acari: Penthaleidae). Crop Pasture Sci. 69, 216–221 (2018).
Grimm, M. A., Hyder, M. B., Doyle, P. B. & Michael, P. C. The effect of pasture feed on offer in spring on pest populations and pasture production. Proc. Aust. Soc. Anim. Prod. 20, 233–236 (1994).
Batz, Z. A. et al. Rapid adaptive evolution of the diapause program during range expansion of an invasive mosquito. Evolution (N Y) 74, 1451–1465 (2020).
van Helden, M., Heddle, T., Umina, P. A. & Maino, J. L. Economic injury levels and dynamic action thresholds for Diuraphis noxia (Hemiptera: Aphididae) in Australian cereal crops. J. Econ. Entomol. 115, 592–601 (2022).
Arthur, A. L., Hoffmann, A. A. & Umina, P. A. Challenges in devising economic spray thresholds for a major pest of Australian canola, the redlegged earth mite (Halotydeus destructor). Pest Manag. Sci. 71, 1462–1470 (2015).
Mata, L. et al. Acute toxicity effects of pesticides on beneficial organisms – Dispelling myths for a more sustainable use of chemicals in agricultural environments. Sci. Total Environ. 930, 172521 (2024).
Acknowledgements
The authors acknowledge Elia Pirtle for assistance with figure design and editorial advice, CSIRO for their assistance in digitising old data records, and the Australian Wool Innovation for their support in updating the online TIMERITE® tool. This study was supported by funding from the Grains Research and Development Corporation (CES2010-001RTX) and Meat and Livestock Australia (P.PSH.1283).
Funding
This study was supported by funding from the Grains Research and Development Corporation (CES2010-001RTX) and Meat and Livestock Australia (P.PSH.1283).
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the research. J.M., J.R.S., and C.P. reviewed the literature, and compiled the historical data collected by J.R.S. and C.P.. X.C. conducted field and laboratory research and additional mite dissections. J.M. conducted the analysis and drafted the manuscript. All authors revised and approved the manuscript. Funding by P.U. and J.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maino, J.L., Umina, P.A., Pavri, C. et al. Adapting pest management strategies to changing climates for the redlegged earth mite, Halotydeus destructor. Sci Rep 14, 16939 (2024). https://doi.org/10.1038/s41598-024-67602-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67602-9
- Springer Nature Limited