Abstract
Aporosa cardiosperma is a plant species majorly found in the Indian Western Ghats that belongs to the phyllanthaceae family with ethnobotanical importance. Using a Fourier Transform-Infrared Spectrometer (FT-IR) and Gas Chromatography-Mass Spectrometry (GC–MS) for evaluating leaf extracts of A. cardiosperma, significant functional groups and metabolite constituents were determined, and its total flavonoid, phenol, and tannin content were quantified. Further, its antibacterial efficacy was investigated against microorganisms that cause fish and human disease and are resistant to common antibiotics, including Staphylococcus aureus, Bacillus subtilis, Mycobacterium tuberculosis, Klebsiella pneumoniae, Aeromonas hydrophila, and Pseudomonas aeruginosa. Regarding the outcomes of GC–MS analysis, the primary metabolites in the A. cardiosperma leaf extracts were heneicosane (57.06%), silane (13.60%), 1-heptadecene (10.09%), 3-hexadecene (9.99%), and pentadecane (9.54%). In comparison to other solvents, methanolic extract of A. cardiosperma leaves had increased phenolic, flavonoid, and tannin content; these findings are consistent with in vitro antioxidant potential and obtained that the methanolic extract (100 µg/mL) exhibited the higher percentage of inhibition in DPPH (82.35%), FRAP (86.20%), metal chelating (72.32%), and ABTS (86.06%) antioxidant assays respectively. Similar findings were found regarding the antibacterial efficacy against pathogenic bacteria. Comparatively, to other extracts, methanolic extracts showed more significant antibacterial activity at a lower minimum inhibitory concentration (MIC) value (250 µg/mL), whilst ethyl acetate and hexane solvent extracts of A. cardiosperma leaves had higher MIC values 500 µg/mL and 1000 µg/mL respectively. The antimicrobial potential was validated by investigating bacterial growth through the extracts acquired MICs and sub-MICs range. Bacterial growth was completely inhibited at the determined MIC range. In conclusion, A. cardiosperma leaf extract's phytochemical fingerprint has been determined, and its potent antibacterial and antioxidant activities were discovered. These findings of the current study will pave the way for developing herbal treatments from A. cardiosperma for various fish and human diseases.
Similar content being viewed by others
Introduction
Medications with various chemical compositions can cause adverse effects, from mild discomfort to severe outcomes. The Food and Drug Administration (FDA) monitors and takes action to ensure safety and report adverse effects1. Herbal medicines are eco-friendly, safe, and offer diverse benefits: antioxidants, antibacterial, anti-inflammatory, and anticancer properties2, that widely utilized in folk heals to treat a range of ailments, such as inflammatory, neoplastic, diabetic, hypertensive, and cardiovascular ailments3. Medicinal plants provide compounds for innovative pharmaceuticals. The phyllanthaceae family, with more than 1000 species worldwide, holds significant potential as an antioxidant, anthelmintic, antibacterial, anti-diarrheal, anti-tumor, anti-inflammation, and insecticidal4. A. cardiosperma, abundantly found in the Western Ghats of South India, provides carbohydrates and mineral-rich fruit. Its mucilage, and tannin-rich cells protect various pests5. Aporosa genus including A. lindleyana6, A. wallichii7, and A. octandra8 are used in various medicinal applications. Plant metabolites are used in aquaculture for nutrition, health, water quality, disease control, and environmental sustainability benefits. Aquaculture is the fastest-growing sector in food production worldwide, supplying 50% of global fish consumption9. Disease emergence in freshwater aquaculture is a significant concern by pathogens like S. aureus, B. subtilis, M. tuberculosis, K. pneumoniae, P. aeruginosa, and A. hydrophila can cause contagious diseases in fish and humans10, when exposed to antibiotics, these pathogens develop resistance, posing risks to humans and the environment11. To overcome this limitation, plant-based drugs, which have been used historically as immune boosters, offer aquaculture-safe, natural alternatives to antibiotics12. Interest in these plants has surged globally due to their simplicity, affordability, and minimal adverse effects on ecosystems and animal life13. Over 30,000 antimicrobial compounds from plants and 1340 species with antibacterial properties have been identified14. Between 14 and 28% of diverse plant species are predicted to possess therapeutic qualities, with 74% of their bioactive compounds explored for medical applications15. Antibacterial resistance stems from widespread, inappropriate antibiotic use, rendering many drugs ineffective16. The World Health Organization (WHO) observed a significant obstacle to medical progress, emphasizing the need for alternative antibiotics to curb resistance17. Researchers are urged to isolate and identify novel bioactive compounds from plants to combat microbial resistance in aquaculture. Still, many medicinal plants possess antibacterial properties are undiscovered; efforts focus on developing rapid, advanced, and efficient treatments13. With this evidence, this study investigates the medicinal plant A. cardiosperma for its antibacterial and antioxidant properties by evaluating the phytochemical composition and metabolites to uncover its potential therapeutic applications.
Materials and methods
Materials required
Solvents used in the experiments are methanol (99%, CDH, New Delhi, India), ethyl acetate (99.5%, Loba chemie, Mumbai, India), and hexane (85%, Molychem, Mumbai, India). Chemicals and reagents required are sulfuric acid (98%, Merck, Mumbai, India), sodium hydroxide (97%, Rankem, Maharashtra, India), chloroform (99%, Loba chemie, Mumbai, India), dragendorff’s reagent (Loba chemie, Mumbai, India), benedict reagent (qualitative, Loba chemie, Mumbai, India), copper sulphate (99%. Himedia, Mumbai, India), aluminum chloride (98%, Merck, Mumbai, India), gallic acid (98%, SRL, Maharashtra, India), folin ciocalteu reagent (Himedia, Mumbai, India), sodium carbonate (99.5%, Merck, Mumbai, India), sodium nitrate (98%, Merck, Mumbai, India), tannic acid (Rankem, Maharashtra, India), quercetin (99%, SRL, Maharashtra, India), DPPH (95%, SRL, Maharashtra, India), ascorbic acid (99.7%, SRL, Maharashtra, India), iron-ferrozine (Biosystem, Tamil Nadu, India), trichloroacetic acid (98%, Fisher scientific, Mumbai, India), potassium ferricyanide (99%, Fisher scientific, Mumbai, India), ferric chloride (98%, Loba chemie, Mumbai, India), ABTS (98%, Himedia, Mumbai, India), potassium persulphate (Himedia, Mumbai, India), ethanol absolute (99.9%, CSS, Jiangsu, China), nutrient agar (Himedia, Mumbai, India), nutrient broth (Himedia, Mumbai, India), streptomycin (95%, Himedia, Mumbai, India), DMSO (99.05%, Himedia, Mumbai, India), resazurin dye (Himedia, Mumbai, India).
Plant collection
The leaves of A. cardiosperma were collected from the Achankovil, Kollam district, Kerala, India. Sample plant situated within geographic coordinates 76° 58′ 08.3″ E–W latitude and 9° 03′36.4″ N–S longitudes. Field studies and experimental research on the methods of collecting medicinal plant material were conducted under the guidelines and regulations provided by the National Medicinal Plants Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India (2009). A. cardiosperma grows in nature abundantly and does not require permissions. Taxonomist Dr. M.U. Sharief, Scientist ‘F’ and Head of Office, Ministry of Environment, Forest and Climate Change, Botanical Survey of India (BSI), Southern Regional Centre, Coimbatore, Tamil Nadu, India, has verified the species and deposited in the institutional Herbarium (Herbarium deposition No: BSI/SRC/5/23/2023/Tech-617).
Leaf extracts of A. cardiosperma
Fresh plant leaves were washed by rinsing them with water, allowed to dry in the shade for two weeks, and then milled into the fine powder. The dry powdered leaf was extracted using various solvents based on polarity, including polar (methanol), mid-polar (ethyl acetate), and non-polar (hexane), for two days of shaking in the shaker. Then, the solvents are filtered and concentrated by evaporating solvents using a hot-air oven for 3 days at 40 °C temperature18. The resultant extracts were stored at 4 °C for further studies.
Phytochemical screening of A. cardiosperma leaf extracts
The dried A. cardiosperma leaves powder, weighing 10 g, was mixed with 100 mL of the aformentioned solvents and filtered for the phytochemical analysis. Such as tannins, phlobatannins, saponins, flavonoids, phenolic flavonoids, terpenoids, steroids, phytosteroids, phenol, alkaloids, carbohydrates, and protein are present in various extracts of A. cardiosperma leaves investigated using Arumugam et al.18,19 methods.
Total phenol content (TPC)
Intially, 0.5 mL of A. cardiosperma leaf prepared extracts were taken and diluted with 8 mL of distilled H2O. Then, 0.5 mL of Folin Ciocalteu reagent (1 N) was added and kept at 40 °C for 10 min. Further, 1 mL of sodium carbonate (20%) was added and kept in the dark for 1 h. The absorbance was read at 765 nm using a UV visible spectrophotometer Synergy HT Multimode Reader19 (Biotek, Winooski, USA). The same procedure was repeated for all standard gallic acid solutions, and the standard calibration curves were obtained. The sample concentration was calculated as gallic acid equivalent (GAE)/50 g.
Total tannin content (TTC)
Folin-Ciocalteu's phenol reagent was used to quantify the total tannin content of A. cardiosperma leaf extracts by the Arumugam et al.19 procedure. In 100 μL of the leaf extract, 8.3 mL of distilled H2O and 0.5 mL of Folin-Ciocalteu phenol reagent were added in a test tube and incubated for 5 min at 37 °C. Then, 1 mL of 35% Na2CO3 solution was mixed in the test tube. The mixture was shaken gently and undisturbed at 25 ± 2 °C for 30 min. The absorbance was measured at 725 nm, and distilled H2O was used as the blank. Tannic acid served as the standard reference, and the total tannin content of the extracts was reported in mg of tannic acid equivalent (TAE)/50 g.
Total flavonoid content (TFC)
Intially, 0.5 mL of the prepared leaf extracts of A. cardiosperma were diluted with 3.5 mL of distilled H2O, followed by the addition of 0.3 mL of 5% sodium nitrate to the tube. After 5 min, the tube was added with 0.3 mL of 10% aluminum chloride. The mixture dissolved with 2 mL of sodium hydroxide (1 M) in the 6th min. The contents of the reaction mixture were thoroughly combined and promptly diluted with 2.4 mL of distilled H2O. The mixtures were read at 414 nm with the comparison to a blank. Quercetin was the standard compound used to quantify total flavonoids and expressed as mg of quercetin equivalent (QE)/50 g20.
FT-IR analysis
The functional groups of the A. cardiosperma leaf extracts were investigated utilizing the KBr pellet method with spectra of 4000–400 cm−1 through an FT-IR spectrophotometer (Perkin Elmer, USA)19.
GC–MS metabolite profiling
Preparation of A. cardiosperma leaf extracts
The proportion of A. cardiosperma leaves was shade dried out, and then 10 g of the powdered leaf was mixed with 100 mL of solvents (methanol, ethyl acetate, and hexane), respectively. Using a Soxhlet apparatus, the solvents were evaporated under reduced pressure at 55–60 °C and dried in a vacuum. The residue was filtered and concentrated into a dry mass by a rotary vacuum evaporator. Then, extracts were filtered and loaded in the glass vial using a sterile syringe filter with a 0.22 µm pore size18.
GC–MS analysis of A. cardiosperma leaf extracts
Gas chromatography–mass spectrometry analysis (GC–MS—QP-2010 Plus, Shimadzu, Tokyo, Japan) was performed on the solvents containing A. cardiosperma leaf extracts using the thermal desorption (TD) system 20. The GC–MS system operated under specified conditions, which included an RTX-5MS capillary column (30 m × 0.25 mm with a film thickness of 0.25 mm), 1.5 mL/min of helium gas (serving as a carrier), 250 °C for the gun, 290 °C for the detector, and 60–180 °C for the column at a rate of 5 °C/min, followed by 180–280 °C at 10 °C/min (10 min), 0.5 scan/s of m/z 40 and 350, and a split ratio of 1:200. One microliter of the prepared sample was used as the injector volume. To avoid the noise peaks, the solvents were passed through the column before the sample injection19. Along with retention indices, the results were cross-checked against previously published literatures using the Wiley 08 spectral Library Searching Programme (http://www.sisweb.com/software/ms/wiley-search.html) and NIST mass 17 spectrum libraries.
In-vitro antioxidant activity of A. cardiosperma leaf extracts
In the present study, the absorbance of the control solution [substrate (DPPH, FRAP, ABTS, Metal chelating) in solvent without leaf extracts of A. cardiosperma] was adjusted to zero to represent the absorbance of the blank solution during the antioxidant tests. Using the formula (Eq. 1), which determines the percentage of inhibition, whereas Atest represents the absorbance of the reaction mixture containing A. cardiosperma leaf extracts/standard, Acont represents the absorbance of the control solution21. Reduced absorbance was denoted by the significance of higher free-radical scavenging capacity.
DPPH radical-scavenging assay
This experiment assessed the A. cardiosperma leaf extracts capacity to scavenge free radicals, following the detailed protocol provided by Brand-Williams et al.22. The procedure involved mixing 1.0 mL of 0.1 mM DPPH in methanol with 1.0 mL of various concentrations of A. cardiosperma leaf extracts (20–100 µg/mL), shaking thoroughly, and then the mixture was kept undisturbed in the dark for 30 min at room temperature. The absorbance of the mixture was measured at 517 nm. Ascorbic acid was used as the reference standard. The percentage of inhibition for DPPH radical scavenging activity was calculated using the Eq. (1).
FRAP assay
The reducing power of A. cardiosperma leaf extracts was ascertained using the El Jemli et al2 approach. This experiment involved mixing different concentrations of A. cardiosperma leaf extracts (20–100 µg/mL) with 2.5 mL of phosphate buffer (200 mM, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was then incubated at 50 ºC for 20 min, followed by rapid cooling. After adding 2.5 mL of 10% TCA (trichloroacetic acid) to the mixture, centrifuging it for 10 min at 3000 rpm. The top layer was removed and added with the same amount of distilled H2O. Finally, the prepared solution was mixed with 1 mL of 1% ferric chloride (FeCl3) and read at 700 nm. An increase in ferric reducing power was inferred from the reaction mixture's increased absorbance. Ascorbic acid, the standard was prepared using the same procedure. The percentage of reducing power was calculated using Eq. (1).
Metal-chelating assay
They were investigating the metal-chelating activity of A. cardiosperma leaf extracts and standard antioxidants using the Dinis et al.23 approach. The different extract concentrations (20–100 µg/mL) were added to 0.05 mL of 2 mM FeCl2; the mixture was left to incubate at room temperature for 5 min. The reaction began with the addition of ferrozine (5 mM; 0.3 mL). The mixture was shaken vigorously after an incubation of 10 min at room temperature. Ascorbic acid served as a positive control. The absorbance was read at 562 nm. Equation (1) was used to calculate the inhibition percentage of the formation of the Fe2+-ferrozine complex.
ABTS radical scavenging assay
The antioxidant qualities of various A. cardiosperma leaf extracts were assessed using the ABTS radical cation decolorization test18. In deionized water, 88 μL of 140 mM potassium persulphate solution was used to dissolve 7 mM ABTS. The mixture was undisturbed for 14 h at 37 °C in the dark. The ABTS mixture was diluted in ethanol (1:89 v/v) to achieve an absorbance. Then, 200 μL of freshly prepared ABTS+ solution was mixed with 100 μL of solvent extracts at different concentrations (20, 40, 60, 80, and 100 μg/mL) and incubated for 10 min. The absorbance of the solution mixtures was read at 734 nm. The standard employed was ascorbic acid. Equation (1) was used to calculate the ABTS radical scavenging activity.
In-vitro antibacterial efficacy of A. cardiosperma leaf extracts
Agar well diffusion method
In-vitro antibacterial activity of A. cardiosperma extracts was evaluated using the agar well diffusion method24. The Microbial Type Culture Collection and Gene Bank (MTCC), located in Chandigarh, India, provided the pure culture of the bacterial strains. These bacterial stains, including gram positive [S. aureus (MTCC 96), B. subtilis (MTCC 736), and M. tuberculosis (clinical isolate)] and gram negative [A. hydrophila (MTCC 646), K. pneumoniae (MTCC 109), and P. aeruginosa (MTCC 2488)] were cotton-swabbed on the freshly prepared nutrient agar plates. A cork borer was used for the gel puncture to make 6 mm diameter wells on nutrient agar plates. Various A. cardiosperma solvent extract concentrations (250, 500, 750, and 1000 μg/mL) were added to the corresponding wells along with positive control [streptomycin (25 μg/mL)]. Then, the petri plates were incubated for 24 h at 37 °C, then the zone of inhibition (mm) was measured. The experiments were carried out in triplicates.
Minimum inhibitory concentration
To estimate the MIC for each A. cardiosperma leaf extracts, a sterile 96-well microtiter plate containing resazurin as a cell growth indicator was utilized18. After sterilizing the laminar air flow chamber, a 96-well microtiter plate, 100 μL of freshly prepared nutrient broth, and 100 μL of various extracts (10 mg/mL). DMSO [10% (v/v)] was added as control without A. cardiosperma leaf extracts to the third row of the plate. Then, 10 μL of resazurin dye and 10 μL of inoculum of bacterial strains (5 × 106 CFU/mL) was added to each well. After that, the microtiter plate was carefully wrapped with an aluminum foil to avoid the dehydration of the bacterial culture and it was kept at 37 °C for 24 h. Visual inspection of the wells revealed that color shifts from purple to pink (positive) or colorlessness were considered negative. All the experiments were carried out in triplicates.
Bacterial growth kinetics
Using a modified Arumugam et al.19 protocol, the growth of S. aureus, B. subtilis, M. tuberculosis, A. hydrophila, K. pneumoniae, and P. aeruginosa were investigated using the determined MIC and sub-MIC (1/2 MIC) of A. cardiosperma leaf extracts. Extracts were added based on the obtained MIC and sub-MIC values (in triplicates) after microbial cultures were added into tubes to achieve a final inoculum of 1.6 × 104 CFU mL−1, and the culture inoculated plates were maintained at 37 °C. Using a UV–visible spectrophotometer (Synergy HT Multimode Reader, Biotek equipment, Winooski, VT, USA), the bacterial growth was measured spectrophotometrically for 24 h at 2-h intervals by measuring the optical density at 600 nm. The streptomycin (25 µg/mL) was used as the positive control, and the well-untreated bacterial cultures acted as the negative control.
Data analysis
All the experiments were carried out in triplicates, and the results were expressed in the mean ± standard error of the mean (SEM). Applying one-way ANOVA with Dunnett's multiple comparisons test25 and two-way ANOVA with Tukey's multiple comparisons test26 on the data was analyzed using GraphPad Prism version 8 (GraphPad Software, Inc., San Diego, CA). The data were presented as descriptive statistics through tables and graphs. *, **, ***, and **** indicate P-values of respectively ≤ 0.05, ≤ 0.01, ≤ 0.001, and ≤ 0.0001 and “ns” indicates no significant.
Results
Phytochemical screening of A. cardiosperma leaf extracts
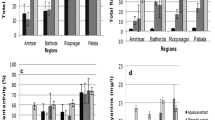
The phytochemical analysis of A. cardiosperma leaf used polar solvents (aqueous, methanol and ethanol) extracts revealed tannins, phlobatannins, saponins, flavonoids, phenolic flavonoids, terpenoids, steroids, phytosteroids, phenols, alkaloids, carbohydrate, and proteins. In contrast, the mid-polar solvents (ethyl acetate, acetone) extract revealed tannins, phlobatannins, saponins, flavonoids, phenolic flavonoids, terpenoids, steroids, phytosteroids, phenols, alkaloids, carbohydrates, and proteins. The non-polar solvents (petroleum ether and hexane) extract contained tannins, phlobatannins, saponins, flavonoids, phenol, steroids, and carbohydrates (Table 1). The total phenolic quantity was found to be higher in methanolic extract (21.61 ± 1.111 mg/GAE g extract), followed by hexane and ethyl acetate extracts of A. cardiosperma leaf (20.02 ± 1.997 mg/GAE g and 3.86 ± 0.703 mg/GAE g) (Fig. 1). Total tannin content of methanolic extract shows higher quantity (36.45 ± 0.793 mg/TAE g extract) compared to the hexane (24.89 ± 0.249 mg/TAE g) and ethyl acetate (19.28 ± 0.471 mg/TAE g extract) respectively. The total flavonoid content of the methanolic is higher (11.47 ± 0.309 mg/QE g), followed by ethyl acetate (3.82 ± 0.519 mg/QE g) and hexane (1.53 ± 0.191 mg/QE g).
Standard graph of Gallic acid equivalents (a), Quantitative analysis of the total phenolic content (b), Standard graph of Tannic acid equivalents (c) and Quantitative analysis of the total tannin content (d), Standard graph of Quercetin equivalents (e), Quantitative analysis of the total flavonoid content (f) present in the various extracts of A. cardiosperma leaf.
Characterization of A. cardiosperma leaf extracts using FT-IR spectroscopy
The FT-IR spectra of A. cardiosperma leaf extracts obtained in the 4000–500 cm−1 region is shown in Fig. 2. The range peaks were identical in all the extracts. Methanolic extract displayed peaks at 3390.86 cm−1, 2978.93 cm−1, 1611.66 cm−1, 1521.71 cm−1, 1443.88 cm−1, 1386.12 cm−1, 1251.20 cm−1 and ethyl acetate extract peaks over 3397.90 cm−1, 2978.48 cm−1, 1758.62 cm−1, 1615.1 cm−1, 1458.88 cm−1, 1383.05 cm−1, 1244.89 cm−1. Hexane extract showed peaks at 2919.66 cm−1, 2851.64 cm−1, 1735.4 cm−1, 1462.05 cm−1, 1379.06 cm−1, 1177.49 cm−1. Strong peaks with high-intensity bands were visible at about 3390.86 cm−1, 3397.90 cm−1 and 2919.66 cm−1 because of the stretching of phenols and alcohols. The peaks for the stretching bends of N–H (amines and amide), C–H (alkanes), and 2978.93 cm−1, 2978.48 cm−1, and 2851.64 cm−1. The peaks corresponding to alkene (C=C stretching) and ketone (C=O stretching) are 611.66 cm−1, 1758.62 cm−1, and 1735.4 cm−1, respectively. In peaks on 1521.71 cm−1, 1458.88 cm−1 and 1462.05 cm−1 indicate C≡C (aromatic) bend, 1386.12 cm−1, 1383.05 cm−1 and 1379.06 cm−1 represent stretching bend C–N bend (amine). The peaks over 1251.20 cm−1, 1244.89 cm−1 and 1177.49 cm−1 for stretching bend of S=O (sulfinyl).
GC–MS analysis of A. cardiosperma leaf extracts
The chromatogram generated by the GC–MS analysis (Fig. 3 displays the percentage peaks in tandem with their retention time) of the methanolic leaf extract recognized 19/20 compounds as biologically potential (Table 2). A total of 28 compounds were found in the ethyl acetate extract, 26 of which are biologically active (Table 3). There were 40 compounds in the hexane extract; 36 are biologically active compounds (Table 4). Heneicosane (57.06%), silane, 9h-fluoren-9-ylidenebis [trimethyl (13.60%), phytol (1.51%) in the methanolic extract, 1-heptadecene (10.09%),3-hexadecene (9.99%), neophytadiene (7.66%) in ethyl acetate extract and pentadecane (9.54%), heneicosane (18.21%), octacosane, 2-methyl (14.28%) in the hexane extract. These bioactive compounds are identified based on peak area percentage and metabolic potential from A. cardiosperma leaf extracts.
In-vitro antioxidative capacity of A. cardiosperma leaf extracts
DPPH radical scavenging assay
Aporosa cardiosperma leaf extracts could scavenge free radicals using DPPH assay and obtained contrast outcomes with the ascorbic acid as standard (Fig. 4a). Ascorbic acid and different solvent extracts both demonstrated a notable dose-dependent reduction of DPPH radicals. However, ascorbic acid's ability to scavenge radicals outperformed and compared with these extracts. Remarkably, at 100 µg/mL, the extracts of methanol, ethyl acetate, and hexane showed increased radical-scavenging percentages of 82.35 ± 0.151%, 73.26 ± 0.361%, and 70.88 ± 0.100% respectively (Fig. 4a). The IC50 value for methanol extract was 28.05 µg/mL, ethyl acetate was 57.7 µg/mL, and hexane was 67.71 µg/mL. Standard ascorbic acid with d an IC50 value of 11.91 µg/mL.
DPPH radical scavenging activity of different extracts of A. cardiosperma leaf (a). FRAP activity of different extracts of A. cardiosperma leaf (b). The metal chelating activity of various extracts of A. cardiosperma leaf (c). ABTS radical scavenging activity of different extracts of A. cardiosperma leaf (d). Data are the means ± standard error of the mean of three replicates. Different superscript asterisk indicates a significant difference between treatments, and “ns” indicates no significance (P < 0.05, Tukey’s multiple comparisons test).
FRAP assay
Based on this, the FRAP assays an electron transfer mechanism of hydrogen ions from the phenolic flavonoid compounds present in the extracts of A. cardiosperma leaf. The ferric dropping power of the A. cardiosperma extracts compared with ascorbic acid (Fig. 4b); however, reducing power by ethyl acetate (71.15 ± 0.231%) and hexane (56.26 ± 0.385%) extracts was inferior to that by methanolic extract (86.20 ± 0.117%) in 100 µg/mL concentrations.
Metal chelating assay
The metal chelating activity of A. cardiosperma leaf extracts revealed that methanol extract showed a higher percentage of inhibition (72.32 ± 0.548%) in 100 µg/mL concentrations (Fig. 4c)compared to ethyl acetate (65.91 ± 0.429%) and hexane extracts (62.17 ± 1.84%). The antioxidant compounds have been found abundantly in the methanol extract, which increases the binding affinity to Fe ions and forms a metal complex that prevents the formation of the Fe ions-ferrozine complex.
ABTS radical scavenging assay
Testing the A. cardiosperma leaf extracts for radical scavenging activities using ABTS. Methanol extract has an elevated percentage of inhibition (86.06 ± 0.217%) compared to ethyl acetate (71.95 ± 0.42%) and hexane (67.26 ± 0.217%) in 100 µg/mL concentrations (Fig. 4d). The IC50 values of hexane was 63.53 µg/mL, ethyl acetate was 44.6 µg/mL, and methanol extract was 21.25 µg/mL. The IC50 value of control ascorbic acid was 10.5 µg/mL. Based on the polarity of the solvents, A. cardiosperma leaf elucidates that the metabolite due to polar solvents has more secondary metabolites that reduce the ROS elevation. A. cardiosperma leaf extract's ability to quench ABTS+ radicals act as a hydrogen donor and could inhibit the oxidation process by shifting free radicals into stable forms.
In-vitro antibacterial activity of A. cardiosperma leaf extracts
Agar well diffusion method
The antibacterial activity was examined in S. aureus, B. subtilis, M. tuberculosis, A. hydrophila, K. pneumoniae, and P. aeruginosa against different solvent extracts of A. cardiosperma leaf (Table 5). Compared to other solvents, methanol extract showed an increased zone of inhibition in all the tested pathogens. From the results obtained, the decrease in solvents polarity also decreased the antibacterial activity. Methanol extracts significant compounds present in the GC–MS analysis with potential antibacterial activity.
Minimum inhibitory concentration
The MIC of the various solvent extracts from A. cardiosperma leaf was shown in Table 6. From the results, methanol has a potential lowest MIC value against tested microorganisms. Methanol shows minimum inhibitory concentration at 250 µg/mL concentrations in all the tested microorganisms. Additionally, ethyl acetate and hexane extracts had MICs of about 500 and 1000 µg/mL, respectively.
Bacterial growth kinetics
These findings suggest that the leaf extracts of A. cardiosperma and their effects on the growth of gram positive bacteria S. aureus, B. subtilis, M. tuberculosis were shown in Fig. 5a–c and gram negative A. hydrophila, K. pneumoniae, and P. aeruginosa are shown in Fig. 6a–c. The MIC and sub-MIC values of A. cardiosperma leaf extract were determined to examine the bacterial growth curve, along with control samples (untreated). A slight difference in growth patterns between the treated and the untreated microorganisms was observed at dosages below the MIC. However, the growth was interrupted at the MIC level. According to this investigation, various solvents in leaf extracts from A. cardiosperma may be able to stop the examined microbial cultures from growing at the MIC level but not at the sub-MIC level.
Growth curves of S. aureus (a) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). Growth curves of B. subtilis (b) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). Growth curves of M. tuberculosis (c) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). All the experiments were compared with positive control: Streptomycin (25 µg/mL).
Growth curves of A. hydrophila (a) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). Growth curves of K. pneumoniae (b) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). Growth curves of P. aeruginosa (c) under the influence of various extracts of A. cardiosperma leaf Methanol (i), Ethyl acetate (ii), and Hexane (iii). All the experiments were compared with positive control: Streptomycin (25 µg/mL).
Discussion
Phytochemical constituents of plant extracts decide their therapeutic activity. Because of this, methanol and water are solvents with higher quantities of phytochemicals27. Phenolics and flavonoids predominantly function as potent antioxidant agents and scavenge harmful free radicals, playing critical therapeutic roles in treating many inflammatory illnesses, cancer, cardiovascular diseases, and diabetes, modulating human health, and metabolism28. Another significant bioactive compound, tannins, has been shown to have antimicrobial, antiviral, antiparasitic, anti-ulcer, and neuroprotective properties29. The high quantity of polyphenolic compounds enhanced antioxidant activity since more active molecules are available to scavenge the available free radicals30. TPC of 107.8 μg GAE/mL for methanolic Impatiens tinctoria tuber extract28, 199.26 mg GAE/g dry weight (DW) for aqueous-methanolic pomegranate leaf extracts31, and 101.38 mg GAE/g DW for aqueous Aegle marmelos fruit extract32 were reported, which shows potential antioxidant and antibacterial activity. TFC of 43.50 mg QE/g DW for aqueous Siamese senna leaf extract32, 136.7 ± 0.04 μg QE/mL for methanolic Impatiens tinctoria tuber extract28, 21.53 mg/g DW for Cassia tora leaf extract33 were reported. Flavonoids are essential for metal chelation, free radical scavenging, stabilization of scavenging compounds, and inhibiting enzymes responsible for free radical production28. TPC and TFC, as the antioxidant activity of the A. cardiosperma leaf extract, were evaluated and found to be significant. TTC of 5.6 mg GAE/g for methanolic Amaranthus lividus leaf extract29 and 21 ± 1.21 mg TAE/g for ethanolic Caulerpa racemose extract were reported, which act as antibacterial agents.
Leaf extract from A. cardiosperma contains aromatic compounds, primary and secondary amines, and alkanes, which are indicators of alkaloids presence by an FT-IR analysis. Artemisia annua leaf has shown similar peaks in medicinal plants like stretching alcohols and phenols34 (3500–32,000 cm−1), indicating that the phenol and flavonoids could be the dominant compounds. Those functional groups carbonyl (C=O) and aromatic tertiary amine, as shown by (1371.39 cm−1) C–N stretch, constitute flavonoid32. Based on the solvent polarity, functional groups of the extract are identified19. Discerning distinct spectra for hexane, ethyl acetate, and methanol was possible. Each solvent contains hydroxyl and ketone groups, but only methanol and ethyl acetate possess alcoholic groups and aromatic amines. These functional groups are essential components of various secondary metabolites, including alkaloids, flavonoids, terpenoids, polyphenols, and tannins35.
The biologically active compounds are abundantly present in methanolic extract. Heneicosane is a significant compound present in the GC–MS analysis of methanol extracts. Heneicosane was found to exhibit excellent antimicrobial activity36. Silane also exhibits antimicrobial activity37. Ethyl acetate extract contains major compounds 1-heptadecene and 3-hexadecene. Yoon et al.38 reported that 1-heptadecene exhibited antifungal activity against Plutella xylostella larvae. Multiple therapeutic characteristics of 3-hexadecene have been reported to treat diabetes, inflammatory illnesses, and cancer20. Hexane extract contains major compounds like pentadecane and heneicosane. According to Bruno et al.39, pentadecane has an antibacterial effect on the parasites of Leishmania infantum. Another study found that pentadecane, a key ingredient in Kaempferia galanga, has strong antimicrobial properties against fungus, gram positive, and gram negative bacteria40.
In-vitro radical scavenging assays such as DPPH and ABTS are used to analyze the effects of A. cardiosperma leaf solvent extracts. The capacity of the target compounds to donate hydrogen ions or scavenge proton radicals is the crucial mechanism of both the antioxidant assays28. According to Durhan et al.29, the addition of various tannins, phenolics, and flavonoids enhances the free radical scavenging activity of Amaranthus lividus leaf extract, which is 75.6% of DPPH scavenging activity at 4 mg/mL concentration. 93.46% of DPPH scavenging activity at 640 µg/mL concentration of Tetrapleura tetraptera fruit extract reported by Okechukwu et al.41. Methanolic Impatiens tinctoria tuber extract shows 62.8% DPPH scavenging activity at 400 µg/mL concentration28. Methanolic Cyperus scariosus leaf extract shows DPPH scavenging activity with an IC50 value of 2.78 ± 0.21 mg/mL previously reported42. Also, polar solvents have more antioxidant potential than non-polar solvents, which agrees with our findings. Methanolic Ficus carica latex extract showed the highest ABTS radical scavenging activity, 98.81 ± 0.34%, among 18 different cultivars43. The FRAP assay increases the absorbance of the reaction mixture to determine the Fe3+–Fe2+ transition44.
A-OH denotes the flavonoid and phenolic functional groups in the A. cardiosperma leaf extracts. Samuel et al.45 observed that the DPPH, ABTS and FRAP activities in the medicinal plant Astragalus membranaceus leaves and roots had the most significant activity, respectively. In the metal-chelating assay, an antioxidant uses an electron transfer pathway to decrease a Fe (III) salt. Copper and iron are transitional metals that increase oxidative stress. Metal ions activity can be reduced through chelation, which will minimize the production of ROS46. The A. cardiosperma methanolic extract may have a higher hydrogen-donating capacity than the ethyl acetate and hexane extracts, contributing to its higher chelating power. Aydin et al.46 reported Salvia officinalis leaf extract shows 82.8% metal chelation activity at 200 mg/mL concentration. Anand et al.47 also mentioned that the Ocimum basilicum medicinal plant methanolic extract shows a higher metal chelating capacity of 82.84% at 100 µg/mL concentration.
A. cardiosperma leaf extracts include a variety of phytochemical agents that have antibacterial properties, including steroids, diterpenes, tannins, flavonoids, saponins, and phenolic compounds. Methanol extract possesses antibacterial activity more than other solvents, when the polarity of the solvent increases, it also increases the zone of inhibition, which indicates that the extracts active secondary metabolite content is concentrated more in the polar parts44. Nguyen et al.48 found that methanolic and ethanolic extracts from the piper beetle Linn exhibited antibacterial effects on gram positive and gram negative microbes. According to Tambe et al.49, Syzygium cumini methanolic extract was effective against gram negative and gram positive bacteria. The gram positive bacteria (Streptococcus pyogenes and S. aureus) and gram negative bacteria (Escherichia coli and P. aeruginosa) were the four bacteria against which the methanolic extract of Pereskia bleo leaves showed potential antibacterial properties50. The hydrophilic or lipophilic character of a chemical affects its antibacterial action. Gram negative bacteria are more resistant to a considerably limited number of lipophilic substances because of their lipid-rich cell walls. Lipopolysaccharide molecules comprise its outer membrane, creating a hydrophilic environment that defends against hydrophobic chemicals41. This mechanism suggests that gram negative microbial species in the present study were more strongly suppressed by methanol extracts and its water component, which contains more hydrophilic compounds51. The antibacterial activity of a plant metabolite is mediated by disruption of microbial membranes, weakening of cellular processes, regulation of biofilm formation, inhibition of bacterial capsule synthesis, and decreased generation of microbial toxins52. Heneicosane was identified as the primary component present in the methanol leaf extract of A. cardiosperma by GC–MS analysis. According to Vanitha et al.36, heneicosane had antibacterial efficacy at different doses towards the bacteria Streptococcus pneumoniae, M. tuberculosis, Bacillus cereus, Salmonella enteritidis, and Acinetobacter baumannii. Priyadarshi et al.53 found that heneicos-1-ene (C21H42) had effective antibacterial action against S. typhi and E. coli at various doses. Cardiospermum halicacabum54 and Macaranga barteri55 both report neophytadiene compounds possessing antibacterial effectiveness. Durio zibethinus methanol extract56 and Solanum macrocarpum Linn ethyl acetate extract57 are the possible candidates for antibacterial action due to 1-heptadecane constituents. The presence of 3-hexadecene, in the seaweed Caulerpa racemosa20 displayed their potential antibacterial properties.
The effectiveness of an infection therapy is influenced by the choice of a therapeutic approach, which is significantly influenced by a reliable estimate of MIC13. A. cardiosperma leaf extracts with an interesting substantial MIC, excluding hexane, compared to methanol and ethyl acetate solvent extracts. These MIC results agreed with early plant extract investigations. According to Ralte et al.35, gram positive and negative bacteria were susceptible to different parts of Parkia timoriana methanolic extracts with MICs ranging from 2.19 to 7.3 mg/mL. According to Johari and Khong50, Pereskia bleo extracts were effective in four examined microorganisms: S. aureus, S. pyogenes, P. aeruginosa, and E. coli. The methanolic extract exhibited significant activity against gram positive and gram negative bacteria, with MIC value of 225 µg/mL and 450 µg/mL, respectively. Ethanolic Calpurnia aurea leaf crude extract showed MIC against P. aeruginosa, S. pneumoniae, and S. aureus was 2.5 mg/mL27.
The bioactive chemicals interact in the microbial cell membrane to restrict the development of microbes19. The subsequent interactions facilitated the active substance to permeate further the cellular cytoplasm, including the nearby protein and nucleic acids, which ultimately led to the execution of bacterial cell death52. Plant polyphenols are thought to have antibacterial effects, primarily via interfering with the function of bacterial cell membranes, which slows bacterial growth or reproduction41. In simple solutions, H2O2 exhibits notable chemical stability. Still, it swiftly undergoes a reaction with ferrous iron, generating highly reactive hydroxyl radicals (·OH), hydroxide anions (–OH), and oxidized ferric iron via the Fenton method reaction58. Numerous biomolecules, including DNA, undergo diffusion-limited reactions with the hydroxyl radical. The primary mechanism of H2O2-induced cell death is DNA damage due to hydroxyl radicals, most likely formed by contact with ferrous iron loosely associated with DNA59. The bioactive effect of the Rosmarinus officinalis and Artemisia monosperma leaf extracts was reported by Soliman et al.52 to have inhibited the development of S. aureus, S. typhimurium, Shigella sonnei, Micrococcus leutus, Clostridium perfringens, and E. coli. This was attributed to the presence of polyphenolic chemicals in the plant. Othman et al.60 reported inhibiting bacterial growth curves by extracts from two Duabanga grandiflora and Acalypha wilkesiana on E. coli and S. aureus. Calpurnia aurea leaf crude ethanol extract has suppressed the growth of P. aeruginosa, S. pneumoniae, and S. aureus, according to the MIC values27.
Conclusion
Compared to other extracts, the methanolic extract, in particular, exhibited superior efficacy in both antioxidant assays and antibacterial activity against antibiotic-resistant pathogens. This could be the presence of the critical metabolites in the methanolic extract and the higher content of phenolic compounds, flavonoids and tannins of A. cardiosperma that neutralize the free radicals. These findings suggest that A. cardiosperma leaf extracts have substantial potential for developing herbal treatments for fish and human diseases. The phytochemical fingerprint and the potent biological activities of these extracts highlight their therapeutic potential, providing a foundation for further research into their mechanisms of action and applications in ethnobotanical medicine. This study paves the way for utilizing A. cardiosperma to develop natural, plant-based remedies for combating oxidative stress and bacterial infections.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
Wei, L. et al. Data mining and analysis for emicizumab adverse event signals based on the Food and Drug Administration Adverse Event Reporting System database. Int. J. Clin. Pharm. 45, 622–629 (2023).
El Jemli, M. et al. Radical-scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Pharm. Sci. 2016, e6392656 (2016).
Ghatti, S. S. et al. A comprehensive review on Sophora japonica: Ethnomedicinal, phytochemistry & pharmacological aspects. Nat. Prod. J. 14, 1–18 (2024).
Kumara, K. K. S., Shishupala, S. & Prakash, H. S. The genus Phyllanthus: A rich source of pharmacologically active compounds useful in traditional and modern medicines. In Ethnic Knowledge and Perspectives of Medicinal Plants (Apple Academic Press, 2023).
Schot, A. M. Systematics of Aporosa (Euphorbiaceae). Blumea. Supplement 17, 1–380 (2004).
Ramakrishnan, S. & Venkataraman, R. Screening of antioxidant activity, total phenolics and gas chromatography-mass spectrophotometer (GC-MS) study of ethanolic extract of Aporosa lindleyana Baill. Afr. J. Biochem. Res. 5, 360–364 (2011).
Shahriar, K. R., Salekin, S., Islam, Q. S., Ahsan, M. & Hasan, C. M. Phytochemical and biological studies on bark extract of Aporosa wallichii Hook f. Dhaka Univ. J. Pharm. Sci. 19, 139–143 (2020).
Vabeiryureilai, M., Lalrinzuali, K., Rosangkima, G. & Chandra, G. Qualitative phytochemical analysis and antioxidant activity of Aporosa octandra (Buch.-Ham. ex D. Don) extracts. Int. J. Pharm. Res. 6, 69 (2014).
Boyd, C. E. et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 51, 578–633 (2020).
Irshath, A. A., Rajan, A. P., Vimal, S., Prabhakaran, V.-S. & Ganesan, R. Bacterial pathogenesis in various fish diseases: Recent advances and specific challenges in vaccine development. Vaccines 11, 470 (2023).
Okaiyeto, S. A. et al. Antibiotic resistant bacteria in food systems: Current status, resistance mechanisms, and mitigation strategies. Agric. Commun. 2, 100027 (2024).
Faheem, M., Hoseinifar, S. H. & Firouzbakhsh, F. medicinal plants in Tilapia aquaculture. In Novel Approaches Toward Sustainable Tilapia Aquaculture (eds. Hoseinifar, S. H. & Van Doan, H.) 161–200 (Springer International Publishing, 2023). https://doi.org/10.1007/978-3-031-38321-2_7.
Liang, H. et al. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 10, 21416 (2020).
Badr, M. M. & Quiroz, I. V. Antimicrobial effect of natural products against bacteria, fungi, and yeasts. Biotechnol. Drug Dev. Target. Hum. Dis. 9, 137–164 (2024).
Vaou, N., Stavropoulou, E., Voidarou, C., Tsigalou, C. & Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 9, 2041 (2021).
Ahmed, S. K. et al. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2, 100081 (2024).
Organization, W. H. WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation. (2018).
Arumugam, M. et al. GC–MS based metabolomics strategy for cost-effective valorization of agricultural waste: Groundnut shell extracts and their biological inhibitory potential. Waste Biomass Valor. 13, 4179–4209 (2022).
Arumugam, M. et al. Comprehensive metabolite profiling and therapeutic potential of black gram (Vigna mungo) pods: Conversion of biowaste to wealth approach. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-022-02806-5 (2022).
Palaniyappan, S., Sridhar, A., Kari, Z. A., Téllez-Isaías, G. & Ramasamy, T. Evaluation of phytochemical screening, pigment content, in vitro antioxidant, antibacterial potential and GC-MS metabolite profiling of green seaweed Caulerpa racemosa. Mar. Drugs 21, 278 (2023).
Manikandan, D. B. et al. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng. 9, 104845 (2021).
Brand-Williams, W., Cuvelier, M.-E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–30 (1995).
Dinis, T. C., Madeira, V. M. & Almeida, L. M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315, 161–169 (1994).
Manikandan, D. B. et al. Biofabrication of ecofriendly copper oxide nanoparticles using Ocimum americanum aqueous leaf extract: Analysis of in vitro antibacterial, anticancer, and photocatalytic activities. Environ. Sci. Pollut. Res. 28, 33927–33941 (2021).
Dunnett, C. W. Multiple comparisons between several treatments and a specified treatment. In Linear Statistical Inference (eds. Caliński, T. & Klonecki, W.) vol. 35 39–47 (Springer New York, 1985).
Keselman, H. J. & Rogan, J. C. The Tukey multiple comparison test: 1953–1976. Psychol. Bull. 84, 1050 (1977).
Wasihun, Y., Alekaw Habteweld, H. & Dires Ayenew, K. Antibacterial activity and phytochemical components of leaf extract of Calpurnia aurea. Sci. Rep. 13, 9767 (2023).
Gidamo, G. H. Antioxidant activity and mineral content of Impatiens tinctoria A. Rich (Ensosila) tuber, an Ethiopian medicinal plant. Sci. Rep. 13, 14998 (2023).
Durhan, B., Yalçın, E., Çavuşoğlu, K. & Acar, A. Molecular docking assisted biological functions and phytochemical screening of Amaranthus lividus L. extract. Sci. Rep. 12, 4308 (2022).
Haq, S. H. et al. Antioxidant, anticancer activity and phytochemical analysis of green algae, Chaetomorpha collected from the Arabian Gulf. Sci. Rep. 9, 18906 (2019).
Yu, M., Gouvinhas, I., Rocha, J. & Barros, A. I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 11, 10041 (2021).
Wongsa, P., Phatikulrungsun, P. & Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 12, 6631 (2022).
Kumar, V. & Roy, B. K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 8, 10714 (2018).
Akgeyik, A. U., Yalçın, E. & Çavuşoğlu, K. Phytochemical fingerprint and biological activity of raw and heat-treated Ornithogalum umbellatum. Sci. Rep. 13, 13733 (2023).
Ralte, L., Khiangte, L., Thangjam, N. M., Kumar, A. & Singh, Y. T. GC–MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci. Rep. 12, 3395 (2022).
Vanitha, V. et al. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod. 154, 112748 (2020).
Yoshino, N. et al. Synthesis and antimicrobial activity of quaternary ammonium silane coupling agents. J. Oleo Sci. 60, 429–438 (2011).
Yoon, M.-Y. et al. Antifungal activity of polyacetylenes isolated from Cirsium japonicum roots against various phytopathogenic fungi. Ind. Crops Prod. 34, 882–887 (2011).
Bruno, F. et al. Cytotoxic screening and in vitro evaluation of pentadecane against Leishmania infantum promastigotes and amastigotes. J. Parasitol. 101, 701–705 (2015).
Tang, X., Xu, C., Yagiz, Y., Simonne, A. & Marshall, M. R. Phytochemical profiles, and antimicrobial and antioxidant activities of greater galangal [Alpinia galanga (Linn.) Swartz.] flowers. Food Chem. 255, 300–308 (2018).
Okechukwu, Q. N., Ugwuona, F. U., Ofoedu, C. E., Juchniewicz, S. & Okpala, C. O. R. Chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin from Monodora myristica and Tetrapleura tetraptera in Southeast Nigeria. Sci. Rep. 12, 19861 (2022).
Gandhi, Y. et al. Chemoprofiling and medicinal potential of underutilized leaves of Cyperus scariosus. Sci. Rep. 14, 7263 (2024).
Shahinuzzaman, M. et al. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci. Rep. 10, 10852 (2020).
Adedayo, B. C. et al. Phytochemical composition and antioxidant properties of methanolic extracts of whole and dehulled Bambara groundnut (Vigna subterranea) seeds. Sci. Rep. 11, 14116 (2021).
Samuel, A. O. et al. Antioxidant and antibacterial insights into the leaves, leaf tea and medicinal roots from Astragalus membranaceus (Fisch.) Bge. Sci. Rep. 11, 19625 (2021).
Aydin, D., Yalçin, E. & Çavuşoğlu, K. Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci. Rep. 12, 15845 (2022).
Anand, T., Sundararajan, M., Anbukkarasi, M., Thomas, P. A. & Geraldine, P. A methanolic extract of Ocimum basilicum exhibits antioxidant effects and prevents selenite-induced cataract formation in cultured lenses of Wistar rats. Pharm. J. 11, 496–504 (2019).
Nguyen, L. T. T., Nguyen, T. T., Nguyen, H. N. & Bui, Q. T. P. Simultaneous determination of active compounds in Piper betle Linn. leaf extract and effect of extracting solvents on bioactivity. Eng. Rep. 2, e12246 (2020).
Tambe, B. D., Pedhekar, P. & Harshali, P. Phytochemical screening and antibacterial activity of Syzygium cumini (L.)(Myrtaceae) leaves extracts. Asian J. Pharm. Res. Dev. 9, 50–54 (2021).
Johari, M. A. & Khong, H. Y. Total phenolic content and antioxidant and antibacterial activities of Pereskia bleo. Adv. Pharmacol. Pharm. Sci. 2019, (2019).
Mogana, R., Adhikari, A., Tzar, M. N., Ramliza, R. & Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med. Ther. 20, 55 (2020).
Soliman, M. M., Elsaba, Y. M., Soliman, M. S. A. & Ahmed, E. Z. Composition and antimicrobial activity of Rosmarinus officinalis L. and Artemisia monosperma L. leaf essential oils and methanolic extracts from plants grown in normal and saline habitats in Egypt. Sci. Rep. 14, 7342 (2024).
Priyadarshi, S., Harohally, N. V., Roopavathi, C. & Naidu, M. M. Isolation, identification, structural elucidation and bioactivity of Heneicos-1-ene from Coriandrum sativum L. foliage. Sci. Rep. 8, 17414 (2018).
Jeyadevi, R., Sivasudha, T., Ilavarasi, A. & Thajuddin, N. Chemical constituents and antimicrobial activity of indian green leafy vegetable Cardiospermum halicacabum. Indian J. Microbiol. 53, 208–213 (2013).
Ogundajo, A., Okeleye, B. & Ashafa, A. O. Chemical constituents, in vitro antimicrobial and cytotoxic potentials of the extracts from Macaranga barteri Mull-Arg. Asian Pac. J. Trop. Biomed. 7, 654–659 (2017).
Zamakshshari, N. H. et al. Chemical profile and antimicrobial activity of essential oil and methanol extract from peels of four Durio zibethinus L. varieties. Biomass Conv. Bioref. 13, 13995–14003 (2023).
Sodipo, O. A., Abdulrahman, F. I., Alemika, T. E., Gulani, I. A. & Akinniyi, J. A. Gas Chromatography-Mass Spectrometry (GC-MS) analysis and antimicrobial investigation of the ethyl acetate extract of “Gorongo”-Solanum macrocarpum L. J. Pharm. Bioresour. 7, 164–172 (2010).
Frawley, E. R. & Fang, F. C. The ins and outs of bacterial iron metabolism. Mol. Microbiol. 93, 609–616 (2014).
Faulkner, M. J. & Helmann, J. D. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid. Redox Signal. 15, 175–189 (2011).
Othman, M. et al. Optimal methods for evaluating antimicrobial activities from plant extracts. J. Microbiol. Methods 84, 161–166 (2011).
Acknowledgements
The authors gratefully thank UGC-SAP-DRS-II (F.3-9/2013[SAP-II], Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST)-Level-I (stage-II) (Ref. No. SR/FST/LSI-647/2015(C) Date.11.08.2016) and Department of Science and Technology Promotion of University Research and Scientific Excellence (DST PURSE Phase—II) (Ref. No. SR/PURSE PHASE 2/16(G) /& 16(C) Date. 21.02.2017) of the Department of Animal Science, Bharathidasan University for the instrumental facility. The authors also thank “RUSA, 2.0-Biological Sciences, Bharathidasan University” Tiruchirappalli. The authors extended their appreciation to the Researchers Supporting Project number (RSP2024R35), King Saud University, Riyadh, Saudi Arabia.
Funding
There is no external funding received to carry out the experiments.
Author information
Authors and Affiliations
Contributions
U.A.: Investigation, methodology, data curation, writing—original draft. D.B.M.: Investigation, resources. M.A.: Methodology, resources. S.Y.A.: Editing, data curation, funding. S.M.: Editing, resources, funding. R.T.: Conceptualization, project administration, supervision, validation, visualization, writing—review & editing. All the authors contributed to the article and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdul, U., Manikandan, D.B., Arumugam, M. et al. GC–MS based metabolomic profiling of Aporosa cardiosperma (Gaertn.) Merr. leaf extracts and evaluating its therapeutic potential. Sci Rep 14, 16010 (2024). https://doi.org/10.1038/s41598-024-66491-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66491-2
- Springer Nature Limited