Abstract
Mortality of patients hospitalized with COVID-19 has remained high during the consecutive SARS-CoV-2 pandemic waves. Early discrimination of patients at high mortality risk is crucial for optimal patient care. Symmetric (SDMA) and asymmetric dimethylarginine (ADMA) have been proposed as possible biomarkers to improve risk prediction of COVID-19 patients. We measured SDMA, ADMA, and other L-arginine-related metabolites in 180 patients admitted with COVID-19 in four German university hospitals as compared to 127 healthy controls. Patients were treated according to accepted clinical guidelines and followed-up until death or hospital discharge. Classical inflammatory markers (leukocytes, CRP, PCT), renal function (eGFR), and clinical scores (SOFA) were taken from hospital records. In a small subgroup of 23 COVID-19 patients, sequential blood samples were available and analyzed for biomarker trends over time until 14 days after admission. Patients had significantly elevated SDMA, ADMA, and L-ornithine and lower L-citrulline concentrations than controls. Within COVID-19 patients, SDMA and ADMA were significantly higher in non-survivors (n = 41, 22.8%) than in survivors. In ROC analysis, the optimal cut-off to discriminate non-survivors from survivors was 0.579 µmol/L for SDMA and 0.599 µmol/L for ADMA (both p < 0.001). High SDMA and ADMA were associated with odds ratios for death of 11.45 (3.37–38.87) and 5.95 (2.63–13.45), respectively. Analysis of SDMA and ADMA allowed discrimination of a high-risk (mortality, 43.7%), medium-risk (15.1%), and low-risk group (3.6%); risk prediction was significantly improved over classical laboratory markers. We conclude that analysis of ADMA and SDMA after hospital admission significantly improves risk prediction in COVID-19.
Similar content being viewed by others
Introduction
Infection with the newly emerged SARS-CoV2 has afflicted millions of people around the globe during the COVID-19 pandemic that lasted from March 2020 to May 2023. This pandemic evolved with several major waves of infection, during which the pathogenicity of the virus underwent several changes over time due to mutations in the virus genome, as reviewed by Nugent1. The COVID-19 disease took a mild to moderate course in the majority of those infected; however, some patients were hospitalized due to severe symptoms and a comparatively high proportion of hospitalized patients did not survive their disease. The pandemic caused an estimated 18 million deaths worldwide, resulting in a significant excess mortality.
In total, 765 million people worldwide were affected by COVID-19, with an overall mortality rate of 0.9%2. However, the mortality rate of those who suffered from severe COVID-19 requiring hospitalization varied during the three major pandemic waves, reaching about 40–50% in early 2020, about 11% in late 2020, then rising again to around 21% in early 2021 and declining thereafter3.
As routine clinical scores and biomarkers only insufficiently allowed to discriminate between patients at high and low risk of in-hospital mortality, many investigators took efforts to find biomarkers that have better prognostic value when taken early after hospital admission4,5,6. The nitric oxide (NO) pathway is a major regulator of vascular function and immune defense in severe infections and sepsis, mediated by the endothelial and inducible isoforms of NO synthase7. We have previously published data showing that the circulating levels of asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), two methylated variants of the amino acid L-arginine which acts as the natural substrate for enzymatic NO production, are suitable biomarkers for disease severity and outcome in sepsis patients8,9. ADMA directly interferes with NO synthesis by competing with L-arginine for binding to the enzyme’s active site10, whilst both dimethylarginines reduce cellular bioavailability of L-arginine through inhibition of cellular L-arginine transport proteins11. In addition, arginase is another enzyme that consumes L-arginine as a substrate by converting it into L-ornithine, thereby potentially reducing substrate availability for NO synthesis12. Arginase has been shown to be upregulated in sickle cell disease, type 2 diabetes mellitus diseases, and other conditions where it limits NO-mediated vascular relaxation13,14.
In a pilot study of 33 hospitalized patients during the first pandemic wave, we found that sequential analysis of ADMA and SDMA in blood samples taken on the first day of hospitalization for COVID-19 significantly improved the ability to predict in-hospital mortality as compared to traditional risk scores and biomarkers like SOFA, leukocyte count, C-reactive protein, and pro-calcitonin15. Subsequently, other investigators corroborated our findings with different analytical techniques16,17,18, but also in small patient cohorts. In addition, in a collaborative study with Italian researchers, we recently reported that severely affected COVID-19 patients with low pulmonary vasodilation detected by chest CT scan could be identified by high plasma ADMA concentration; these patients had a poor outcome19. By contrast, COVID-19 patients with severe clinical disease but with high pulmonary vasodilation showed ADMA levels comparable to those with mild disease as well as better outcome. This data suggests that there might be a pathophysiological role of dimethylarginines in controlling pulmonary blood flow during SARS-CoV2 infection; their addition to the clinical laboratory marker spectrum might enhance identification of high-risk COVID-19 patients. In the present study, we investigated the roles of ADMA and SDMA as predictive biomarkers of COVID-19-related in-hospital mortality in a multicenter study involving four University medical centers in Germany and a large number of COVID-19 patients. In addition, we analyzed a broader spectrum of L-arginine-related metabolites including L-ornithine, the product of arginases, and L-citrulline, the product of the NO synthesis pathway, in order to receive a more complete picture of the disturbances in L-arginine biochemical pathways.
Patients and methods/material and methods
Study participants
We identified 394 patients with COVID-19 who were included in one of the four participating biobanks. After exclusion of patients with missing serum samples (N = 59), patients with missing outcome data (N = 155), and patients for whom no serum sample was available from days 1–4 after hospital admission (N = 53), we included 180 patients who were admitted with symptomatic COVID-19 to one of the four participating medical centers between January 02, 2020 and January 22, 2021 (Fig. 1). Patients were included if the main cause for hospitalization was COVID-19; all patients had a positive SARS-CoV-2 PCR test result in respiratory samples analyzed on admission or externally prior to admission. Participating centers were the University Medical Center Hamburg-Eppendorf (UKE), the University Clinic Aachen (UKA), the Medical School of Hannover (MHH), and the Charité Berlin (ChB).
As a control group, we recruited 127 healthy age- and sex-matched blood donors who consented to donate the remainder of a blood sample that was routinely collected for clinical chemistry testing for research purposes. All blood donors had been evaluated for the absence of diseases that prevented them from donating blood, and had consented to participation after written and verbal information.
Study protocol
The entire study protocol was approved by the Ethics Committee of the Hamburg Board of Physicians (2021-100662-BO-ff and 2022-300225-WF). All patients gave their informed consent for their blood samples to be included into the local biobanks. The Ethics Committee of the Medical Faculty of RWTH Aachen had consented the Covid-19 Aachen study (COVAS) according to its vote EK080/20 and the regulations of the RWTH centralized Biomaterial Bank (RWTH cBMB; vote EK206/09). The Ethics Committee of the Hannover Medical School (MHH) had approved the collection of biomaterial and data from COVID-19 patients and the broad molecular characterization of the MWK COVID-19 cohort of the Hannover Unified biobank of MHH (vote number 9001_BO_K). Patients at Chb were included as part of the Pa-Covid-19 study (DRKS00021688) which was approved by the Charité Ethics Committee (EA2/066/20)20. All investigations were performed in accordance with the Declaration of Helsinki in its latest revision. No further patient selection criteria were applied. Serum samples had been collected from all patients and stored in the local biobank frozen at -80°C until analysis of biomarkers. Samples from days 1–4 after admission were available from all patients; in addition, consecutive samples taken during up to 14 days after admission were available from a subgroup of 23 patients. Routine clinical data, laboratory parameters, and parameters of clinical disease course and outcome were collected locally and transmitted for statistical analysis in a pseudonymized manner. All patients were treated according to best medical practice and individual clinical needs. Patients were either isolated under standard care or treated in the intensive care units (ICU). The decision on treatment strategies was based on clinical judgment of the severity of the disease and the presence or absence of acute respiratory distress syndrome (ARDS). Follow-up was continued for all patients until in-hospital death or discharge.
Assessment of biomarkers by UPLC-MS/MS
Validated protocols for liquid chromatography-tandem mass spectrometry (LC–MS/MS) were used to quantify L-arginine, L-ornithine, L-citrulline, ADMA and SDMA concentrations in serum21. Briefly, 25 μL of serum were diluted in methanol to which stable isotope labelled internal standards had been added. Subsequently, the compounds were converted into their butyl ester derivatives and quantified by LC–MS/MS (Xevo TQ-S cronos, Waters GmbH, Eschborn, Germany). Compounds were separated on an Aquity UPLC BEH C18 column (2·1 × 50 mm, 1.7 μm, Waters GmbH). The coefficient of variation for the quality control samples was below 15% for all compounds. All biomarkers were measured by investigators blinded to patient outcome.
Assessment of clinical patient status
Patients were assessed as eligible based on a positive RT-PCR test for SARS-CoV-2 in a respiratory tract sample as previously described22. Vital parameters presented in this study were taken between four and 24 h following hospital admission or intubation. The SOFA score was recorded as a measure of clinical patient status23. Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition24. Acute kidney injury was defined according to the AKIN criteria25 and/or need for continuous veno-venous hemofiltration in patients with no pre-existing chronic renal failure. Patients with a body mass index (BMI) of 25 to < 30 kg/m2 were classified as overweight and those with BMI ≥ 30 kg/m2 as obese. Diabetes and prediabetes were defined by clinical history, medication and HbA1c values ≥ 6.5% or ≥ 5.7 to < 6.5%, respectively. Serum and whole blood samples were obtained routinely at the time of admission. Complete blood count, coagulation tests, inflammatory markers [circulating levels of C-reactive protein (CRP), pro-calcitonin (PCT)] and creatinine levels in blood were measured among other tests. Creatinine clearance was estimated using the CKD-EPI formula26.
Statistical analyses
All variables were tested for normal distribution using the Kolmogorov–Smirnov test. Data are presented as mean with standard deviation (SD). Differences between groups were tested for significance using the nonparametric Mann–Whitney U test for two groups or the Kruskal–Wallis analysis of variance for more than two groups. The Chi2 test was used for comparison of categorical variables between groups. Time courses of ADMA and SDMA concentrations were examined using repeated measures two-way ANOVA followed by Tukey’s multiple comparisons test. Spearman’s rank correlation was used to assess pairwise correlations. Survival analyses were performed using Kaplan–Meier curves comparing patients with ADMA and SDMA above or below the cut-off value determined in receiver-operated curve (ROC) analyses. The Youden index was calculated to identify the optimal cut-off for biomarkers27. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by multivariable-adjusted logistic regression analyses. As we had identified two biomarkers, ADMA and SDMA, as predictors of COVID-19 mortality, we analyzed additional models using (SDMA + ADMA) or (SDMA × ADMA) as variables, respectively. In addition, we performed a decision tree analysis to determine risk upon sequential analysis of SDMA and ADMA. Cut-offs to separate risk groups were based on values determined in ROC analysis for both biomarkers. All statistical analyses were performed using SPSS (version 25; IBM Corporation, Armonk, NY, USA) and GraphPad Prism (version 6.01, GraphPad Software, San Diego, CA, USA). For all tests, p < 0.05 was considered statistically significant.
Results
Baseline characteristics
Patients had a mean age of 62.1 ± 15.4 years (range 22–91 years); 64/180 patients (35.6%) were female. Hospital admission was on day 6.7 ± 6.2 after the onset of COVID-19 symptoms. Baseline patient characteristics are shown in Table 1 for the entire cohort and for patients who survived or died during hospitalization. The first blood sample was taken on days 1–4 after hospital admission (mean, day 2.0 ± 1.2).
Mean age of controls was 54.9 ± 11.9 years (range 22–74 years) and 44/127 (34.6%) of controls were women. According to their status as blood donors, none of the control subjects had major chronic diseases, nor fever or any acute signs of infection; their routine laboratory analyses showed no pathological findings. We were unable to include healthy controls for COVID-19 patients older than 74 years, as individuals in the age range above that age were not present amongst blood donors. Thus, matching of COVID-19 patients and health controls for age was incomplete.
Clinical course of the patients
The mean duration of hospitalization was 19.7 ± 20.6 days. 41 patients (22.8%) died during hospitalization. 81 patients (45.0%) were classified as having acute respiratory distress syndrome (ARDS), and were treated in ICU. 51 patients (28.3%) received mechanical ventilation, and 35 patients (19.4%) were treated with ECMO. The rates of ARDS and subsequent ICU treatment, mechanical ventilation, and ECMO therapy were significantly higher in the subgroup of patients who died than in survivors (Table 1). Survivors had lower SOFA scores at admission; also, inflammatory laboratory markers like leukocyte count, CRP, and pro-calcitonin taken at the same time as L-arginine-related biomarkers were significantly higher in patients who died than in survivors (Table 1).
Concentrations of L-arginine-associated biomarkers in COVID-19 patients versus controls
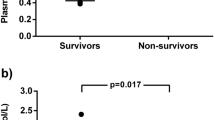
The concentrations of ADMA and SDMA in the first available serum sample after hospital admission were significantly higher in COVID-19 patients than in healthy blood donors (Fig. 2a,b). The serum concentration of L-ornithine was significantly higher and that of L-citrulline was significantly lower in COVID-19 patients than in controls, whilst there was no significant difference in L-arginine concentration (Table 2, supplementary Figure 1). These differences in biomarker concentrations resulted in a significant elevation of the L-ornithine/L-arginine ratio (Orn/Arg Ratio), whilst the L-citrulline/L-arginine ratio (Cit/Arg Ratio) was significantly reduced in COVID-19 patients versus controls (Fig. 2c,d). When we restricted our analysis to fully matched COVID-19 patients and controls, the differences in mean metabolite concentrations remained unchanged (Supplementary Table 1).
Box plots showing the serum concentrations of ADMA (a), SDMA (b), the L-ornithine/L-arginine ratio (c), and the L-citrulline/L-arginine ratio (d) in hospitalized Covid-19 patients who survived or died during hospitalization, as compared to age- and sex-matched healthy controls. Boxes show the median and interquartile range of the data, with whiskers representing the 2.5th to 97.5th percentiles; data points outside of this distribution are plotted individually. Statistical significances were calculated by one-way ANOVA followed by Tukey's multiple comparisons test. ADMA, asymmetric dimethylarginine; Cit/Arg Ratio, L-citrulline/L-arginine ratio; Orn/Arg Ratio, L-ornithine/L-arginine ratio; SDMA, symmetric dimethylarginine.
Within the group of COVID-19 patients, ADMA and SDMA concentrations were significantly higher in patients who died during hospitalization than in survivors (Fig. 2a,b), as was L-ornithine concentration, whereas there were no significant differences in L-arginine and L-citrulline concentrations (Table 2). The Orn/Arg Ratio and the Cit/Arg Ratio showed no significant differences between survivors and non-survivors (Fig. 2c,d).
Associations of ADMA and SDMA with mortality in COVID-19
In univariate logistic regression analyses, ADMA and SDMA were significantly associated with survival (ADMA, OR 63.051 (9.122–435.821), p < 0.001; SDMA; OR 2.514 (1.410–4.480), p = 0.002). Both biomarkers remained significantly associated with mortality in a model adjusted for age and sex and in a model adjusted for age, sex, and leukocyte count, whilst only ADMA retained statistical significance in a models adjusted for age, sex, and CRP. Both biomarkers showed no significant associations in models adjusted for age, sex, and eGFR or PCT (Table 3).
In receiver-operated curve (ROC) analyses for each compound against mortality, SDMA had an AUC of 0.726 (0.642–0.809; p < 0.001), and ADMA had an AUC of 0.728 (0.638–0.819; p < 0.001) (Fig. 3a,b). The optimal ADMA cut-off concentration to discriminate between fatal cases and survivors was determined as 0.599 µmol/L (sensitivity, 0.780; specificity, 0.626), and the optimal cut-off for SDMA was determined as 0.579 µmol/L (sensitivity, 0.927; specificity, 0.475).
Receiver-operated curve (ROC) charts for SDMA (a) and ADMA (b), and Kaplan–Meier survival curves for SDMA (c) and ADMA (d). The serum concentration allowing optimal discrimination between Covid-19 survivors and non-survivors is marked by arrows in (a) and (b); survival curves were constructed by splitting data at these pre-determined concentrations. ADMA, asymmetric dimethylarginine; AUC, area under the curve; OR, odds ratio; SDMA, symmetric dimethylarginine.
Using these cut-off values, we performed Kaplan–Meier analyses. Patients whose serum SDMA concentration or ADMA concentration was above the cut-off concentration had significantly higher mortality rates than those with serum SDMA or ADMA equal to or below the respective threshold concentration. The odds ratio for COVID-19-associated death in patients with SDMA > 0.579 µmol/L was 11.45 (3.37–38.87; p < 0.0001; Fig. 3c), and the odds-ratio in patients with ADMA > 0.599 µmol/L was 5.95 (2.63–13.45; p < 0.0001; Fig. 3d).
Associations of combined ADMA and SDMA with mortality in COVID-19
Next, we tested whether the combined analysis of SDMA and ADMA improved predictive power using a decision tree algorithm. Sequential measurements of SDMA and ADMA significantly enhanced the discrimination of mortality risk (Fig. 4). Patients with both, high SDMA and high ADMA concentrations had a high mortality risk of 43.7%, as compared to an intermediate risk of 15.1% in patients with either SDMA or ADMA elevated, and a low mortality risk of 3.6% in patients with both ADMA and SDMA low (Fig. 5). The odds ratio for patients in the intermediate risk group versus low risk group was 4.80 (1.03–23.76; p = 0.049); the odds ratio in the high versus low risk group was 20.93 (4.73–92.62; p < 0.0001). In ROC analyses, (ADMA + SDMA) and (ADMA x SDMA) resulted in AUCs of 0.734 (0.648–0.821) and 0.741 (0.654–0.828), respectively (both p < 0.0001) (Supplementary Figure 2).
Kaplan–Meier survival curves for combined analysis of ADMA and SDMA serum concentrations. Both low means both biomarker concentrations below their respective cut-off levels from ROC analysis (see Fig. 2a,b); intermediate means that one biomarker is above and the other is below the respective cut-off level; both high indicates that both biomarkers were above their respective cut-off levels. OR, odds ratio.
Decision tree analysis for assessing the risk of in-hospital mortality based on sequential analysis of SDMA and ADMA. Out of 180 patients, 41 died (22.6%). First decision step: Patients were identified as having elevated risk when SDMA levels were ≥ 0.579 μmol/L, and moderate risk when SDMA levels were < 0.579 µmol/L. Second decision step: Additional analysis of ADMA allowed identification of patients with high risk (SDMA ≥ 0.579 µmol/L and ADMA ≥ 0.599 µmol/L (mortality, 43.7%), intermediate risk (SDMA ≥ 0.579 µmol/L or ADMA ≥ 0.599 µmol/L (mortality, 15.1%), or low risk (SDMA < 0.579 µmol/L and ADMA < 0.599 µmol/L (mortality, 3.6%).
Improved prediction of disease severity with ADMA and SDMA compared to classical inflammatory laboratory markers
The well-established inflammatory laboratory markers, leukocyte cell count, C-reactive protein (CRP), and pro-calcitonin (PCT), as well as the SOFA score at admission were all significantly associated with mortality risk, with the relative risks for total mortality associated with each of these traditional markers lying in the range of 2–threefold (Table 4). Amongst combinations of these classical risk markers, SOFA and leukocyte count, SOFA and PCT, CRP and leukocyte count, and CRP and PCT produced the strongest increases in hazard ratio (Supplementary Figure 3a).
We next tested the combination of traditional laboratory markers and SOFA with SDMA and ADMA (Table 5). SOFA score in combination with ADMA, SDMA, or their combination did not result in improved risk prediction (Supplementary Figure 3b), nor did leukocyte count or PCT (Supplementary Figure 3c,d). By contrast, the combination of CRP with ADMA alone, (ADMA + SDMA), and (ADMA x SDMA) resulted in the greatest increases in hazard ratio (Supplementary Figure 3e). COVID-19 patients with elevated CRP and elevated (ADMA x SDMA) had a hazard ratio for mortality of 10.0 (1.56–64.23), p < 0.0001.
Time course of ADMA and SDMA during clinical treatment in COVID-19 survivors and non-survivors
Consecutive blood samples from 23 COVID-19 patients (12 survivors, 11 non-survivors) were available for analysis. The serum concentrations of SDMA and ADMA significantly increased in non-survivors during the first 14 days in hospital, i.e., in the period before their death. By contrast, both biomarkers showed stable concentrations over time in survivors (Fig. 6a–d). As a consequence, the L-arginine/ADMA ratio significantly decreased over time in non-survivors (Supplementary Figure 4a). The time course of the L-citrulline/L-arginine ratio showed no clear differentiation between survivors and non-survivors (Supplementary Figure 4b), whereas we observed a significant increase in L-ornithine/L-arginine ratio over time in non-survivors (Supplementary Figure 4c).
Time course of serum concentrations of SDMA and ADMA in Covid-19 patients who survived (a,c) or died (b,d) during hospitalization. The coloured lines indicate the groups’ means and standard deviations at each time point (blue, survivors, red, non-survivors). Dotted horizontal lines in plots (b) and (d) mark the time that elapsed until the day of death of COVID-19 non-survivors. ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine.
Discussion
Our study provides evidence from a multicenter study that quantification of ADMA and SDMA in blood samples taken as early as possible after admission of COVID-19 patients to hospital contribute significantly to enhanced estimation of patients’ mortality risk during in-hospital treatment. Whilst each of the two biomarkers alone had limited prognostic value and was not superior to classical risk determinants in routine clinical chemistry and clinical scoring, addition of either ADMA or SDMA to such traditional risk predictors significantly improved the predictive power. Sequential analysis of both, ADMA and SDMA, allowed discrimination of a high risk, medium risk, and low risk group of COVID-19 patients with greatly different rates of in-hospital mortality. In addition, the time courses of ADMA and SDMA in repetitive blood samples during the first two weeks of hospitalization showed significant dichotomy between survivors and non-survivors, respectively.
The hospital mortality rate in the patient population included in this study was 20.7%, which compares to 29% in our pilot study15, 11% in the study by Sozio and co-workers19, and 22% in another study by Karacaer and co-workers18. Non-survivors in our study were significantly older than survivors, had lower systolic and diastolic blood pressure and higher systemic inflammatory markers, and required more intensive treatment including extracorporeal membrane oxygenation in a high percentage of patients. These differences in clinical characteristics were mirrored by differences in L-arginine-related biomarkers: As compared to controls, COVID-19 patients had higher L-ornithine concentrations, indicative of arginase activation, as well as higher L-citrulline concentrations, indicative of increased NOS enzymatic activity, whereas L-arginine concentrations were not significantly different. L-ornithine concentrations were also significantly elevated in non-survivors as compared to survivors, and the Orn/Arg Ratio significantly increased over time in non-survivors as opposed to survivors.
Most notably, however, the dimethylated L-arginine metabolites, ADMA and SDMA, were elevated in COVID-19 patients compared to controls; both were higher in non-survivors than in survivors, and they showed a further increase in non-survivors during the first two weeks of hospitalization. Taken together, these two biomarkers made it possible to significantly discriminate between COVID-19 patients with low, intermediate, and high mortality risk. Further, these two biomarkers significantly enhanced the predictive power of classical inflammatory markers. Thus, our study proves that ADMA and SDMA serum concentrations are suitable risk markers in severely diseased COVID-19 patients, and they corroborate our previous findings as well as those reported for small patient samples by other investigators. Using an untargeted metabolomics profiling approach in 27 COVID-19 patients compared to 36 healthy controls, Alboniga and co-workers reported significantly elevated ADMA and SDMA concentrations as well as decreased L-citrulline concentrations in COVID-19 patients16. Hasimi and colleagues compared 57 patients with moderately severe COVID-19 disease with 29 patients with severe COVID-19 and 21 healthy controls. They found significantly elevated ADMA in all COVID-19 patients as compared to controls, and a gradual increase in SDMA which was highest in patients with severe COVID-1917. In another study using a sandwich ELISA technique to measure ADMA, Karacaer and colleagues reported a higher ADMA increase in patients with severe COVID-19 than in those with a mild disease course18.
L-arginine metabolism is involved in numerous biochemical processes that play a role during systemic inflammation: L-arginine serves as a substrate for NO synthase (NOS), where the inducible isoform of NOS (iNOS) is involved in unspecific host defense7. At the same time, L-arginine is also the substrate for endothelial NOS, which generates NO by a much smaller catalytic rate and is an important physiological regulator of the homeostasis in vascular tone and vascular function28. From our measurements of L-arginine and L-citrulline, the by-product of NOS, we cannot deduct which isoform of NOS was responsible for the conversion of L-arginine to L-citrulline; therefore, this metabolic ratio remains a rough surrogate measure of total NOS activity—even more so as other biochemical pathways may also affect L-arginine and L-citrulline concentrations. However, the differences in metabolite concentrations between survivors and non-survivors that we noted in our study are suggestive of higher NOS activity and higher arginase activity in patients who died of COVID-19. Upregulation of inducible iNOS is commonly seen in sepsis patients, where excessive NO production contributes to low blood pressure which may lead to septic shock29. Interestingly, arterial blood pressure was lower in patients who died than in survivors within our multicenter cohort, supporting the hypothesis that iNOS induction may have contributed to their fatal outcome.
L-arginine can also be converted to L-ornithine by either of two isoforms of arginase30. The Orn/Arg Ratio serves as a metabolic surrogate marker of arginase activity; however, the metabolic flux of L-ornithine to, e.g., polyamines or collagen, also determines its levels. Whilst arginase-1 is highly expressed in hepatocytes, red blood cells, and immune cells, arginase-2 is a more ubiquitous enzyme that can be upregulated by inflammatory cytokines30. Therefore, we speculate that the systemic inflammation present in those COVID-19 patients that ultimately did not survive hospitalization may have contributed to higher arginase activity and, thus, higher Orn/Arg Ratio in our study.
The core result of our study is that ADMA and SDMA are elevated in hospitalized COVID-19 patients at the time of hospitalization. Although our study is limited by the fact that we were unable to include healthy controls of high age, the finding that the levels of ADMA and SDMA were even higher in patients with high risk of mortality and further increased in non-survivors as compared to survivors strongly suggest that the differences we measured between groups are meaningful. In the quest for more reliable risk predictors of COVID-19-associated mortality, our previous pilot study as well as a small number of other studies with small patient numbers suggested that ADMA and SDMA may be suitable for risk prediction in COVID-19. We confirm these prior observations here in a multicenter study design reporting that cut-off values of 0.599 µmol/L for ADMA and 0.579 µmol/L for SDMA are optimal discriminators of high- and low-risk COVID-19 patients during their hospitalization.
Our study is limited by the fact that the control group was matched by age and sex, but not for underlying health conditions. Thus, some of the difference in ADMA and SDMA concentrations between COVID-19 patients and healthy controls may have been due to the difference in mean age and the absence of chronic diseases like chronic lung disease, heart diseases, and diabetes mellitus in the control group. To this end, we performed a supplementary analysis restricted to 127 fully age-matched patients and controls, in which we found very closely similar differences in mean biomarker concentrations like in the complete dataset. Therefore, the known age-dependence of plasma ADMA and SDMA concentrations appears not to have had a major impact on the differences in biomarker levels in the present study. In addition, the major comparison in our study was between survivors and non-survivors among COVID-19 patients. There were some differences in the prevalence of co-morbidities between survivors and non-survivors as displayed in Table 1. However, the “net effect” of the co-morbidities on ADMA and SDMA levels is difficult to account for, as, for example, chronic lung disease, which is associated with high ADMA, was more prevalent in non-survivors, whilst cardiovascular disease and chronic kidney disease, which are also associated with high ADMA, were less prevalent in non-survivors.
The mechanism leading to upregulation of SDMA and ADMA concentrations may be related to systemic inflammation. We recently reported associations of these two biomarkers with inflammation in the population-based SHIP cohort31. In previous studies, we had found a strong interrelation between ADMA and CRP in predicting the progression of vascular intimal damage in hemodialysis patients32, and proposed that upregulation of DDAH activity during sepsis might counterbalance induction of iNOS activity33. Accordingly, in sepsis patients, we found that SDMA and ADMA predicted survival in a cohort of 120 ICU-treated patients with sepsis9. In the latter study, SDMA was correlated with pro-calcitonin and creatinine serum levels whilst ADMA was not. Both markers were correlated with lactate levels, which is suggestive of a relation to microcirculatory failure and tissue hypoxia. Finally, our previous study19 demonstrated that amongst COVID-19 patients classified into low, intermediate, and high risk groups by a machine-learning approach, ADMA was highest in the high-risk group and it was associated by absence of pulmonary vasodilation as analyzed in thoracic CT scan. These findings are in line with previous work by our group dissecting the molecular mechanisms of regulation of ADMA and SDMA in pulmonary hypoxia and the role of ADMA in modulating hypoxic pulmonary vasoconstriction34. Clearly, a mechanistic link of SARS-CoV-2 virus to ADMA and SDMA metabolism cannot be established from our clinical cohort study, but the existing data from the present study and the previous studies cited above clearly warrant further research into possible mechanisms. Systemic inflammation and pulmonary hypoxia might be two conditions worth being explored in this context.
In conclusion, our study strongly suggests that adding ADMA and SDMA as biomarkers to our portfolio of diagnostic assessment in hospitalized COVID-19 patients provides significant benefit for early discrimination of patients with a severe, life-threatening disease progression. This may help to better allocate resources to patients at high risk of in-hospital mortality.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Nugent, M. A. The future of the COVID-19 pandemic: How good (or bad) can the SARS-CoV2 spike protein get?. Cells 11(5), 855 (2022).
COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–21. Lancet 399(10334), 1513–1536 (2022).
Gray, W. K., Navaratnam, A. V., Day, J., Wendon, J. & Briggs, T. W. R. COVID-19 hospital activity and in-hospital mortality during the first and second waves of the pandemic in England: An observational study. Thorax 77(11), 1113–1120 (2022).
Qin, J. J. et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension 76(4), 1104–1112 (2020).
Zhao, Z. et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS ONE 15(7), e0236618 (2020).
Altschul, D. J. et al. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci. Rep. 10(1), 16726 (2020).
Cinelli, M. A., Do, H. T., Miley, G. P. & Silverman, R. B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 40(1), 158–189 (2020).
Winkler, M. S. et al. Markers of nitric oxide are associated with sepsis severity: An observational study. Crit Care 21(1), 189 (2017).
Winkler, M. S. et al. Symmetrical (SDMA) and asymmetrical dimethylarginine (ADMA) in sepsis: High plasma levels as combined risk markers for sepsis survival. Crit Care 22(1), 216 (2018).
Böger, R. H. et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 98(18), 1842–1847 (1998).
Banjarnahor, S., Rodionov, R. N., König, J. & Maas, R. Transport of L-arginine related cardiovascular risk markers. J. Clin. Med. 9(12), 3975 (2020).
Morris, S. M. Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br. J. Pharmacol. 157(6), 922–930 (2009).
Morris, C. R. et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama 294(1), 81–90 (2005).
Romero, M. J. et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 102(1), 95–102 (2008).
Hannemann, J. et al. Elevated serum SDMA and ADMA at hospital admission predict in-hospital mortality of COVID-19 patients. Sci. Rep. 11(1), 9895 (2021).
Albóniga, O. E. et al. Metabolic snapshot of plasma samples reveals new pathways implicated in SARS-CoV-2 pathogenesis. J. Proteome Res. 21(3), 623–634 (2022).
Haşimi, A., Doğan, Ö., Serdar, C. C. & Serdar, M. A. Association of serum ADMA, SDMA and L-NMMA concentrations with disease progression in COVID-19 patients. Biochem. Med. (Zagreb). 33(1), 010701 (2023).
Karacaer, C. et al. Association of mortality and endothelial dysfunction with serum ADMA level in COVID-19 patients. Pak. J. Med. Sci. 38(7), 1808–1815 (2022).
Sozio, E. et al. The role of asymmetric dimethylarginine (ADMA) in COVID-19: association with respiratory failure and predictive role for outcome. Sci. Rep. 13(1), 9811 (2023).
Kurth, F. et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19). Infection 48(4), 619–626 (2020).
Schwedhelm, E. et al. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 851(1–2), 211–219 (2007).
Balfanz, P. et al. Early risk markers for severe clinical course and fatal outcome in German patients with COVID-19. PLoS ONE 16(1), e0246182 (2021).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22(7), 707–710 (1996).
Ranieri, V. M. et al. Acute respiratory distress syndrome: the Berlin Definition. Jama 307(23), 2526–2533 (2012).
Mehta, R. L. et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2), R31 (2007).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 150(9), 604–612 (2009).
Youden, W. J. Index for rating diagnostic tests. Cancer 3(1), 32–35 (1950).
Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33(7):829–37, 37a–37d.
Taylor, B. S. & Geller, D. A. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock 13(6), 413–424 (2000).
Caldwell, R. W., Rodriguez, P. C., Toque, H. A., Narayanan, S. P. & Caldwell, R. B. Arginase: A multifaceted enzyme important in health and disease. Physiol. Rev. 98(2), 641–665 (2018).
Winkler, M. S. et al. Association of asymmetric and symmetric dimethylarginine with inflammation in the population-based study of health in pomerania. Biomolecules 13(11), 1612 (2023).
Zoccali, C. et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am. Soc. Nephrol. 13(2), 490–496 (2002).
Böger, R. H. Live and let die: asymmetric dimethylarginine and septic shock. Crit Care 10(6), 169 (2006).
Hannemann, J. & Böger, R. Dysregulation of the nitric oxide/dimethylarginine pathway in hypoxic pulmonary vasoconstriction-molecular mechanisms and clinical significance. Front. Med. (Lausanne). 9, 835481 (2022).
Acknowledgements
We gratefully thank Mariola Kastner for her excellent technical assistance. This work was funded by the Joachim Herz Foundation, Hamburg, Germany. We also thank the Lower Saxony Ministry of Science and Culture (MWK) for funding of the MWK COVID-19 biobank of Hannover Medical School. The PA-Covid-19 study group is acknowledged for providing samples and data for this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.H. and R.B. conceptualized the study, analysed and interpreted the data, applied for funding, and wrote the first draft of the final manuscript. A.Z., Y.M., P.B., E.D., S.V., T.I., E.S., F.K., A.S., M.A., and A.H. contributed to data acquisition and analysis, and critically revised the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hannemann, J., Zink, A., Mileva, Y. et al. A multicenter study of asymmetric and symmetric dimethylarginine as predictors of mortality risk in hospitalized COVID-19 patients. Sci Rep 14, 15739 (2024). https://doi.org/10.1038/s41598-024-66288-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66288-3
- Springer Nature Limited