Abstract
Background
During infection, there is an activation of the L-arginine-nitric-oxide pathway, with a shift from nitric oxide synthesis to a degradation of L-arginine to its metabolites, asymmetric and symmetric dimethylarginine (ADMA and SDMA). However, the prognostic implications for short-term or long-term survival remains unclear. We investigated the association of L-arginine, ADMA, and SDMA with adverse clinical outcomes in a well-defined cohort of patients with community-acquired pneumonia (CAP).
Methods
We measured L-arginine, ADMA, and SDMA in 268 CAP patients from a Swiss multicenter trial by mass spectrometry and used Cox regression models to investigate associations between blood marker levels and disease severity as well as mortality over a period of 6 years.
Results
Six-year mortality was 44.8%. Admission levels of ADMA and SDMA (μmol/L) were correlated with CAP severity as assessed by the pneumonia severity index (r = 0.32, p < 0.001 and r = 0.56, p < 0.001 for ADMA and SDMA, respectively) and higher in 6-year non-survivors versus survivors (median 0.62 vs. 0.48; p < 0.001 and 1.01 vs. 0.85; p < 0.001 for ADMA and SDMA, respectively). Both ADMA and SDMA were significantly associated with long-term mortality (hazard ratios [HR] 4.44 [95% confidence intervals (CI) 1.84 to 10.74] and 2.81 [95% CI 1.45 to 5.48], respectively). The effects were no longer significant after multivariate adjustment for age and comorbidities. No association of L-arginine with severity and outcome was found.
Conclusions
Both ADMA and SDMA show a severity-dependent increase in patients with CAP and are strongly associated with mortality. This association is mainly explained by age and comorbidities.
Trial registration
ISRCTN95122877. Registered 31 July 2006.
Similar content being viewed by others
Background
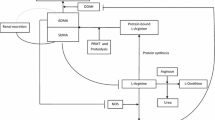
Nitric oxide (NO), which is synthesized from L-arginine by nitric oxide synthases (NOS), is a vaso- and bronchodilator [1]. Its deficiency results in airway hyperreactivity [2] and endothelial dysfunction [3]. In addition, nitric oxide inhibits platelet adhesion and is considered to have a role in nonspecific immunity [4]. Arginase, which converts L-arginine into L-ornithine and urea, reduces the bioavailability of L-Arginine for NOS and thereby inhibits the L-Arginine-Nitric oxide pathway [5]. L-arginine residues are methylated by protein methyltransferases (PRMT). Asymmetric and symmetric dimethylarginine (ADMA and SDMA) are derived from the proteolysis of these methylated arginines on various proteins (Fig. 1) [6, 7]. While ADMA acts as a competitive inhibitor of the NOS, SDMA is a competitor of arginine transport but does not interfere with NOS [8]. Thus, an elevated serum ADMA level results in lowered NO levels, which leads to vasoconstriction, augmented thrombocyte aggregation, and cell adhesion to the endothelium and promotes proliferation of vascular muscle cells [9]. ADMA is metabolized to citrulline and dimethylamine by the enzyme dimethylarginine dimethylaminohydrolase (DDAH) and a small fraction is renally excreted whereas SDMA is almost entirely eliminated by the kidneys. There is also a negative feedback loop of NO, with high NO concentrations leading to the inhibition of DDAH [10].
Metabolism of nitric oxide. Nitric oxide (NO) is synthesized from L-arginine. This reaction is catalyzed by nitric oxide synthases (NOS). Protein synthesis incorporates L-arginine into proteins. Protein-bound L-arginine is methylated by protein methyltransferases (PRMT) and lysed to the metabolites asymmetric and symmetric dimethylarginine (ADMA, SDMA). ADMA is mainly metabolized to citrulline and dimethylamine via dimethylarginine dimethylaminohydrolase (DDAH). A small fraction of ADMA and almost the entire amount of SDMA are renally eliminated. ADMA inhibits the NOS competitively and SDMA interferes with L-arginine transport. Arginase reduces the bioavailability of L-arginine by degrading it to L-ornithine and urea. Nitric oxide has a negative feedback mechanism to DDAH, which leads to less metabolism of ADMA
Circulating levels of ADMA are elevated in multiple diseases with endothelial dysfunction (e.g., hypertension, hyperlipidemia, diabetes mellitus, atherosclerosis, and renal failure) [3, 6, 11–14], in diseases of the respiratory system (e.g., asthma and COPD) [2, 15], and in multiple organ failure in sepsis [16]. Its isomer, SDMA, is also associated with a higher prevalence of cardiovascular risk factors as well as all-cause and cardiovascular mortality [10, 17]. Increased plasma concentrations of ADMA and SDMA are independent prognostic factors for short-term and long-term mortality in critically ill patients [18, 19]. In contrast, high levels of serum SDMA, but not of ADMA, seem to be an independent prognostic biomarker of all-cause mortality in the general population [17]. Both, ADMA and SDMA are increased in elder people [20]. In addition, a lowered ratio of arginine to ADMA is a sensitive risk marker for atherosclerosis, cardiovascular disease, and mortality in patients with shock, being even more sensitive than ADMA alone [21, 22].
Community-acquired pneumonia (CAP) is to date one of the leading causes of death due to an infection [23]. CAP is also associated with an increased risk of cardiovascular events and, thus, is an independent cardiovascular risk factor [24–26]. The aim of our study was to evaluate the role of L-arginine, ADMA, and SDMA in the pathophysiology of CAP. We also wanted to determine whether the ratio of arginine to ADMA correlates with disease severity and mortality in CAP patients over a follow-up period of 6 years.
Methods
Study design
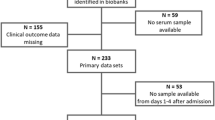
A total of 268 patients with a definite diagnosis of CAP and available blood samples from a previous Swiss multicenter trial (the ProHOSP trial) [27, 28] were included in this investigation. The initial trial included 925 (68%) CAP patients with an all-cause mortality of 45% who were followed up over a period of 6 years [29].
The initial trial was a noninferiority randomized controlled trial evaluating the economical use of antibiotics at six Swiss secondary and tertiary care centers between October 2006 and March 2008 [27]. The primary goal of the study was to verify the efficacy and safety of using serum procalcitonin (PCT) levels to guide the initiation and duration of antibiotic therapy in patients with lower respiratory tract infections [27, 28]. Patients ≥18 years of age presenting from the community or a nursing home to the emergency department (ED), were included if they met at least one of the following criteria: cough, dyspnea, pleural pain, sputum production, or tachypnea and one sign of an infection (core body temperature >38 °C, shivering, white blood cell count >10 or <4x109 cells/L), or one clinical finding upon auscultation (rales or crepitation). An infiltrate was radiologically confirmed in all patients with CAP. Exclusion criteria included language restriction or dementia that precluded patients from giving written informed consent, intravenous drug use, a terminal condition, or hospital-acquired pneumonia (HAP). Participants were accepted into the study if they had received short-term antibiotic pretreatment or corticosteroids, but were excluded if they had obtained long-term antibiotic pretreatment or suffered from severe immunosuppression at ED presentation.
Analysis of blood biomarkers
Blood samples collected from each patient and frozen in aliquots at ED admission were used for measuring the different biomarkers. Baseline arginine, ADMA, and SDMA levels were measured in blood samples of all 268 CAP patients included in the study, with 109 of them also having blood specimens available at day 7 of hospitalization for these measurements. If any of above biomarkers had a value of 0, it was considered to be a measuring error and excluded from the analysis. Due to technical errors or insufficient volume of blood specimens during biomarker measurement, not all patients had a complete set of markers. The final analysis included 268 patients of which 268 (100%) had complete markers of arginine, while 233 (87%) and 242 (90%) had ADMA and SDMA markers available, respectively.
Liquid chromatography tandem-mass spectrometry (LC-MS/MS) analysis was performed using an Ultimate 3000 UHPLC (Thermo Fisher, San Jose, USA) system coupled to an ABSciex 5500 quadrupole mass spectrometer (ABSciex, Darmstadt, Germany) and the AbsoluteIDQ p180 Kit (BIOCRATES Life Sciences AG, Innsbruck, Austria) [30–32]. Sample preparation and measurements were performed as described in the AbsoluteIDQ p180 user’s manual. Chromatographic separation was performed on a Thermo Syncronis aQ 50x2.1 mm 1.7 μm column. The prepared samples were analyzed using multiple reaction monitoring (MRM). Selected metabolites were quantified by reference to appropriate internal standards. All concentrations were reported in μM.
Main outcome measurements
The primary endpoint was all-cause mortality after a follow-up period of 6 years. Secondary endpoints were mortality at day 30 and 1-year, ICU admissions, and clinical findings (pneumonia severity index [PSI] and quick Sepsis Related Organ Failure Assessment [qSOFA] score) [33]. Vital status was ascertained through structured phone interviews by trained medical students at days 30, 180, and 540, and at 6 years [29, 34]. If patients or their household members could not be reached, the treating general practitioners were contacted.
Statistical analyses
All statistical analyses were performed using STATA 12.1 (Stata Corp, College Station, TX, USA). Statistical significance was considered at a p value <0.05. Categorical variables are expressed as percentages (numbers) and continuous variables as medians (interquartile range [IQR]) unless stated otherwise. The distribution of ADMA, SDMA, and L-arginine (just referred to as biomarkers henceforth) were skewed. The distribution of the biomarkers approximated a normal distribution after logarithmic transformation at a base of 10. Chi-square (Wald) tests were used for frequency comparisons, and nonparametric tests (Mann-Whitney U, Kruskal-Wallis, and Spearman’s rank correlation) were executed for comparisons of two or more groups. The significance levels were subjected to Bonferroni adjustment. To study the association of comorbidities with initial biomarker levels, we used univariate and multivariate linear regression models. To investigate associations between biomarker levels at baseline and all-cause mortality, we utilized univariate and multivariate Cox regression models. These associations are reported as hazard ratios (HRs) with 95% confidence intervals (CIs) and significance levels for the chi-square (Wald) test. Odds ratios were calculated and reported with 95% confidence intervals (CIs). Area under the receiver operating characteristics curves (AUCs) with 95% CIs are presented to illustrate discrimination and predictive power. Mortality based on biomarker quartiles (highest versus lower three) has been illustrated through the use of Kaplan-Meier curves. All analyses were performed with the ratio of arginine to ADMA as well.
Results
Patient characteristics
Baseline characteristics of the entire cohort (N = 268) as well as for patients stratified by 6-year survival status are presented in Table 1. The median age of the entire cohort was 72 years and 40.7% were female. There was a high burden of comorbidities, with 18.3% having a history of coronary artery disease (CAD), 23.9% having chonic renal failure (CRF), and 26.1% having chronic obstructive pulmonary disease (COPD).
Association between the investigated biomarkers (L-arginine, ADMA, SDMA) and mortality
Mortality at day 30 was 5.2% (n = 14), increasing to 17.9% (n = 48) after 1 year and 44.8% (n = 120) after 6 years of follow up. Figure 2 shows Kaplan-Meier curves for 6-year all-cause mortality based on the highest quartile compared to lower quartiles of arginine, ADMA and SDMA.
Baseline levels of ADMA and SDMA were increased in 6-year non-survivors compared with survivors (ADMA median, 0.62 μmol/L [IQR 0.47–0.83] vs. 0.48 μmol/L [IQR 0.38–0.67]; p <0.001 and SDMA median, 1.01 μmol/L [IQR 0.63–1.38] vs. 0.85 μmol/L [IQR 0.52–1.00]; p <0.001). There were no significant differences in arginine levels between non-survivors and survivors (median, 41.3 μmol/L [IQR 29.6–62.2] vs. 43.3 μmol/L [IQR 28.2–64.3]; p = 0.97) (see Table 1).
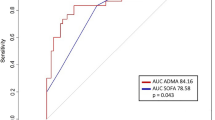
In Cox regression analyses, while ADMA, SDMA, and their ratios were found to be associated with long-term mortality, only SDMA was related to short-term mortality. This association did not remain robust following adjustment for different comorbidities. Further analyses revealed an AUC of SDMA of 0.71 (95% CI 0.56 to 0.87) for short- and mid-term mortality and 0.64 (95% CI 0.57 to 0.71) for long-term mortality at 6 years. The AUCs for ADMA did not reach these levels (Table 2).
Association between biomarkers and severity
In Spearman’s rank correlation analyses with Bonferroni adjustment, we found significant associations of ADMA and SDMA with the Pneumonia Severity Index (ρ = 0.31, p <0.001 and ρ = 0.5307, p <0.001). In contrast, there was no association between all the investigated biomarkers and the qSOFA score.
Association of biomarkers with comorbidities
We developed linear regression models investigating predictors for increased biomarker levels. Univariate analysis showed significant correlation of SDMA and ADMA to numerous comorbidities and patient characteristics (Table 3). The multivariate models revealed that higher levels of SDMA are seen in patients with chronic renal failure, COPD, and in older patients. Levels of SDMA therefore showed a better correlation to comorbidities than ADMA, which was associated only with age and neoplastic disease. Levels of arginine did not show any correlation whatsoever (Table 4).
Discussion
The findings of this first study to investigate arginine, ADMA, and SDMA levels in CAP patients revealed that high levels of ADMA and SDMA measured upon ED admission are strongly associated with mid- and long-term mortality, and that high levels of SDMA are associated with short-term mortality. However, this effect wore off after adjustment for comorbidities and age. That leads us to the assumption that this association is mainly defined by the correlation of the biomarkers to several comorbidities like chronic renal failure or COPD and age. Supporting this assumption is the finding that SDMA is also well correlated with PSI but not with the qSOFA score. In contrast, the other biomarkers were not correlated with any of these scores. Arginine did not show any association with mortality or any secondary outcome.
High levels of SDMA and ADMA are indeed associated with mortality [10, 17–19], but we found that this correlation is mainly defined by comorbidities and age. Several studies have found ADMA and SDMA to be associated with chronic renal failure, COPD, asthma, sepsis, cardiovascular risk factors, and cardiovascular and all-cause mortality [2, 3, 6, 10–17]. In short, these two biomarkers are associated with diseases characterized by vascular and bronchial dysfunction. This association could be causal or caused by an imbalance of nitric oxide production [2, 3]. That does not diminish the prognostic value of these markers, but instead shows that they rather provide an overview of the general health status of a patient. This strong correlation with comorbidities, particularly with renal failure, partly explains the good association with the pneumonia risk scores. Our study transfers these findings to patients with CAP, where an association of ADMA, SDMA, and all-cause mortality was found. In contrast to O’Dwyer et al. we did not find a correlation of these biomarkers with sepsis severity [16]. One reason may be, that we assessed sepsis severity by the qSOFA score and O’Dwyer by the SOFA score, which has more power. The other reason may be the limitation, that we did not assess the qSOFA in the initial trial. So we have to consider that we underestimate the score because of the lack of relevant information in some patients (see below in the limitations).
Two earlier studies found that a lower ratio of arginine to ADMA is a more sensitive risk marker for vascular dysfunction and mortality in shock patients than ADMA alone [21, 22]. In our population, we did not find any advantage of the ratios due to the lack of correlation of arginine with mortality and other outcomes.
Hypertension results in higher levels of ADMA and SDMA [6, 10]. It has also been seen that administration of ADMA results in elevated blood pressure in healthy humans [13]. This suggests that antagonising ADMA or SDMA is a potential new target in the therapy of hypertension or renal failure. One way to reach this goal is L-arginine supplementation. In animal studies, long-term L-arginine supplementation seemed to be safe [35, 36]. In rats it showed an attenuation of angiotensin-II - mediated vasoconstriction [37] but in mice with type 1 diabetes it showed no prevention or reduction of renal injury [38].
Raising arginine levels in critically ill human patients by L-arginine supplementation has shown controversial results relating to safety and benefit [39–43]. A meta-analysis of double-blinded, randomized controlled trials showed a significant lowering of systolic and diastolic blood pressure following supplementation of L-arginine [44]. However, there is still little evidence for supplementation in patients with hypertension and there are no studies that investigate the development of complications and progression of chronic renal failure after administration of L-arginine.
In summary, and in contrast to previous studies, our analysis did not reveal ADMA and SDMA to be independent risk factors for all-cause mortality in CAP patients [10, 17]. However, in agreement with earlier investigations, our study showed that SDMA levels detected herein were strongly and independently associated with renal failure, closely related to the glomerular filtration rate [6], and associated with COPD [2, 15], and that ADMA and SDMA are associated with age. The independent association with age may be due partly to the association of ADMA and SDMA with subclinical atherosclerosis [45, 46].
The well-defined cohort of patients with CAP of different severities is one of the main strengths of this study. It is representative of patients usually treated in hospital EDs. The long follow up over a period of 6 years, the high rate of all-cause mortality, and the highly precise laboratory measurement methods are also worth mentioning. A limitation of our study was that it was carried out in hospitals in Switzerland and the results may not be generalizable to other countries. In addition, autopsies were not performed to validate the cause of death, because of which this study only focused on all-cause mortality. Third, this was only a hypothesis-generating observational study. Fourth, the qSOFA score was not measured at ED admission, as a result of which we had to calculate the score on the basis of the information we had. We took data on confusion from the PSI score and counted it as an altered state of mind. Also not all of our patients had information about the respiratory rate and the others had only an estimated rate. We therefore have to assume that we undervalued the general level of the qSOFA score. We also did not have data on the severity of comorbidities (e.g., we only knew that patients had a chronic kidney disease and not the stage of the kidney disease). We also did not measure NO levels and so cannot be sure that our conclusions are complete. Finally, we did not have a complete marker set available for all patients due to low blood specimen volume. Nevertheless, our hypothesis-generating study provides valuable information that will facilitate the design and conduct of additional studies in this area.
Conclusion
Deducing from our findings, we may assume that neither SDMA nor ADMA nor L-arginine are independently associated with all-cause mortality in CAP patients, although strong unadjusted associations of SDMA—more than ADMA—with mortality and comorbidities are seen in these patients. It remains unclear if higher ADMA and SDMA levels cause higher mortality or if higher mortality causes high levels of ADMA and SDMA, and the precise role NO plays in this scheme of events.
Given our findings, future studies should focus on the causal connection between ADMA and SDMA levels and mortality and on investigating the clinical benefit of antagonising ADMA and SDMA (for example, with L-arginine).
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CAP:
-
Community-acquired pneumonia
- CHF:
-
Congestive heart failure
- CI:
-
Confidence interval
- CRF:
-
Chronic renal failure
- CRP:
-
C-reactive protein
- DDAH:
-
Dimethylarginine dimethylaminohydrolase
- DM:
-
Diabetes mellitus
- ED:
-
Emergency department
- HAP:
-
Hospital-acquired pneumonia
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LC-MS/MS:
-
Liquid chromatography tandem-mass spectrometry
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PCT:
-
Procalcitonin
- PRMT:
-
Protein arginine methyl transferase
- PSI:
-
Pneumonia severity index
- qSOFA:
-
Quick Sepsis related Organ Failure Assessment: altered mental status, fast respiratory rate (≥22 breaths per min), low blood pressure (systolic blood pressure ≤100 mmHg)
- SDMA:
-
Symmetric dimethylarginine
References
Nickler M, Ottiger M, Steuer C, Huber A, Anderson JB, Muller B, Schuetz P. Systematic review regarding metabolic profiling for improved pathophysiological understanding of disease and outcome prediction in respiratory infections. Respir Res. 2015;16:125.
Scott JA, Duongh M, Young AW, Subbarao P, Gauvreau GM, Grasemann H. Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD). Int J Mol Sci. 2014;15(4):6062–71.
Shivkar RR, Abhang SA. Ratio of serum asymmetric dimethyl arginine (ADMA)/ nitric oxide in coronary artery disease patients. J Clin Diagn Res : JCDR. 2014;8(8):CC04–6.
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–12.
Maarsingh H, Pera T, Meurs H. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(2):171–84.
Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7(5):275–85.
Servillo L, Giovane A, Cautela D, Castaldo D, Balestrieri ML. The methylarginines NMMA, ADMA, and SDMA are ubiquitous constituents of the main vegetables of human nutrition. Nitric Oxide Biol Chem / Off J Nitric Oxide Soc. 2013;30:43–8.
Bode-Boger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H. Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am SocNephrol : JASN. 2006;17(4):1128–34.
Ferrigno A, Rizzo V, Bianchi A, Di Pasqua LG, Berardo C, Richelmi P, Vairetti M. Changes in ADMA/DDAH pathway after hepatic ischemia/reperfusion injury in rats: the role of bile. Biomed Res Int. 2014;2014:627434.
Mangoni AA. The emerging role of symmetric dimethylarginine in vascular disease. Adv Clin Chem. 2009;48:73–94.
Pope AJ, Karuppiah K, Cardounel AJ. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res. 2009;60(6):461–5.
Tanhauserova V, Tomandl J, Pacal L, Kleparnik M, Maluskova D, Bartakova V, Kuricova K, Rehorova J, Stepankova S, Svojanovsky J, et al. ADMA, SDMA and L-arginine/ADMA ratio but not DDAH genetic polymorphisms are reliable predictors of diabetic nephropathy progression as identified by competing risk analysis. Kidney Blood Press Res. 2012;36(1):200–8.
Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, Macallister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23(8):1455–9.
Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6(2):82–90.
Scott JA, North ML, Rafii M, Huang H, Pencharz P, Subbarao P, Belik J, Grasemann H. Asymmetric dimethylarginine is increased in asthma. Am J Respir Crit Care Med. 2011;184(7):779–85.
O’Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care. 2006;10(5):R139.
Gore MO, Luneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, Atzler D, de Lemos JA, Grant PJ, McGuire DK, et al. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2013;33(11):2682–8.
Koch A, Weiskirchen R, Bruensing J, Duckers H, Buendgens L, Kunze J, Matthes M, Luedde T, Trautwein C, Tacke F. Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm. 2013;2013:413826.
Pizzarelli F, Maas R, Dattolo P, Tripepi G, Michelassi S, D’Arrigo G, Mieth M, Bandinelli S, Ferrucci L, Zoccali C. Asymmetric dimethylarginine predicts survival in the elderly. Age. 2013;35(6):2465–75.
Atzler D, Schwedhelm E, Nauck M, Ittermann T, Boger RH, Friedrich N. Serum reference intervals of homoarginine, ADMA, and SDMA in the study of health in Pomerania. Clin Chem Lab Med. 2014;52(12):1835–42.
Notsu Y, Yano S, Shibata H, Nagai A, Nabika T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis. 2015;239(1):61–6.
Visser M, Vermeulen MA, Richir MC, Teerlink T, Houdijk AP, Kostense PJ, Wisselink W, de Mol BA, van Leeuwen PA, Oudemans-Van Straaten HM. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J Nutr. 2012;107(10):1458–65.
Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for. Natl Vital Stat Rep Ctr Dis Control Prev, Natl Ctr Health Stat, Natl Vital Stat Syst 2016. 2014;65(4):1–122.
Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, Newman A, Loehr L, Folsom AR, Elkind MS, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–74.
Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125(6):773–81.
Vazquez M, Jockers K, Christ-Crain M, Zimmerli W, Muller B, Schuetz P. MR-pro-atrial natriuretic peptide (MR-proANP) predicts short- and long-term outcomes in respiratory tract infections: a prospective validation study. Int J Cardiol. 2012;156(1):16–23.
Schuetz P, Christ-Crain M, Wolbers M, Schild U, Thomann R, Falconnier C, Widmer I, Neidert S, Blum CA, Schonenberger R, et al. Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC Health Serv Res. 2007;7:102.
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–66.
Alan M, Grolimund E, Kutz A, Christ-Crain M, Thomann R, Falconnier C, Hoess C, Henzen C, Zimmerli W, Mueller B, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Int Med. 2015;278:174–84.
Weinberger KM. Metabolomics in diagnosing metabolic diseases. Therapeutische Umschau Revue Therapeutique. 2008;65(9):487–91.
Yet I, Menni C, Shin SY, Mangino M, Soranzo N, Adamski J, Suhre K, Spector TD, Kastenmuller G, Bell JT. Genetic influences on metabolite levels: a comparison across metabolomic platforms. Plos One. 2016;11(4):e0153672.
Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C, Altmaier E, Kastenmuller G, Kato BS, Mewes HW, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42(2):137–41.
Schuetz P, Koller M, Christ-Crain M, Steyerberg E, Stolz D, Muller C, Bucher HC, Bingisser R, Tamm M, Muller B. Predicting mortality with pneumonia severity scores: importance of model recalibration to local settings. Epidemiol Infect. 2008;136(12):1628–37.
Guertler C, Wirz B, Christ-Crain M, Zimmerli W, Mueller B, Schuetz P. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J. 2011;37(6):1439–46.
Yang Y, Wu Z, Jia S, Dahanayaka S, Feng S, Meininger CJ, Mcneal CJ, Wu G. Safety of long-term dietary supplementation with L-arginine in rats. Amino Acids. 2015;47(9):1909–20.
Hu S, Li X, Rezaei R, Meininger CJ, Mcneal CJ, Wu G. Safety of long-term dietary supplementation with L-arginine in pigs. Amino Acids. 2015;47(5):925–36.
Das S, Mattson DL. Exogenous L-arginine attenuates the effects of angiotensin II on renal hemodynamics and the pressure natriuresis-diuresis relationship. Clin Exp Pharmacol Physiol. 2014;41(4):270–8.
You H, Gao T, Cooper TK, Morris Jr SM, Awad AS. Diabetic nephropathy is resistant to oral L-arginine or L-citrulline supplementation. Am J Physiol Renal Physiol. 2014;307(11):F1292–301.
Bower RH, Cerra FB, Bershadsky B, Licari JJ, Hoyt DB, Jensen GL, Van Buren CT, Rothkopf MM, Daly JM, Adelsberg BR. Early enteral administration of a formula (impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med. 1995;23(3):436–49.
Caparros T, Lopez J, Grau T. Early enteral nutrition in critically ill patients with a high-protein diet enriched with arginine, fiber, and antioxidants compared with a standard high-protein diet. The effect on nosocomial infections and outcome. JPEN J Parenter Enter Nutr. 2001;25(6):299–308. discussion 308–299.
Tepaske R, Velthuis H, Oudemans-Van Straaten HM, Heisterkamp SH, van Deventer SJ, Ince C, Eysman L, Kesecioglu J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: a randomised placebo-controlled trial. Lancet. 2001;358(9283):696–701.
Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, Zanforlin G, Tognoni G. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003;29(5):834–40.
Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34(11):1980–90.
Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162(6):959–65.
Riccioni G, Scotti L, D’Orazio N, Gallina S, Speziale G, Speranza L, Bucciarelli T. ADMA/SDMA in elderly subjects with asymptomatic carotid atherosclerosis: values and site-specific association. Int J Mol Sci. 2014;15(4):6391–8.
Bahls M, Friedrich N, Atzler D, Felix SB, Nauck MA, Boger RH, Volzke H, Schwedhelm E, Dorr M. L-arginine and SDMA serum concentrations are associated with subclinical atherosclerosis in the Study of Health in Pomerania (SHIP). Plos One. 2015;10(6):e0131293.
Acknowledgments
We are thankful to the emergency department, medical clinic, and central laboratory staff of the University Hospital Basel and the Cantonal Hospitals Aarau, Liestal, Lucerne, and Muensterlingen, and the ‘Buergerspital’ Solothurn for their assistance and technical support. In particular, we thank all patients, patients’ relatives, and all local general practitioners who participated in this study. The authors acknowledge the members of the ProHOSP Study Group for their important support.
The ProHOSP Study Group included: U. Schild, K. Regez, R. Bossart, C. Blum, M. Wolbers, S. Neidert, I. Suter, H.C. Bucher, F. Mueller, A. Chaudry, J. Haeuptle, R. Zarbosky, R. Fiumefreddo, M. Wieland, C. Nussbaumer, A. Christ, R. Bingisser, and K. Schneider (University Hospital Basel, Basel, Switzerland); T. Bregenzer, D. Conen, A. Huber, and J. Staehelin (Kantonsspital Aarau, Aarau, Switzerland); W. Zimmerli, C. Falconnier, and C. Bruehlhardt (Kantonsspital Liestal, Liestal, Switzerland); C. Henzen and V. Briner (Kantonsspital Luzern, Luzern, Switzerland); T. Fricker, C. Hoess, M. Krause, I. Lambinon, and M. Zueger (Kantonsspital Muensterlingen, Muensterlingen, Switzerland); and R. Thomann, R. Schoenenberger, and R. Luginbuehl (Buergerspital Solothurn, Solothurn, Switzerland).
Funding
This study was supported in part by the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531 / 1) and the Research Council of the Kantonsspital Aarau (1410.000.044). The initial trial was funded by the Swiss National Science Foundation (grant SNF 3200BO-116177/1), Santé Suisse, the Gottfried and Julia Bangerter-Rhyner Foundation and BRAHMS Biomarkers.
Availability of data and materials
Original data cannot be made public, due to lack of permission from the institutional review board, but are available from the corresponding author on reasonable request.
Authors’ contributions
PS, MC-C, and BM conceptualized and designed the study, wrote the protocol, and initiated the initial ProHOSP study. PS, MAM, MO, PK and AV drafted the present manuscript and performed the statistical analyses. CS, LB, and AH performed laboratory measurements using the p180-kit. AV, MO, MAM, CS, LB, PK, AH, MC-C, CHe, CHo, RT, WZ, BM and PS contributed to the data acquisition, interpretation and drafting of the analyses, critical review for important content, and final approval of the manuscript. PK edited the language of the manuscript. PS had full access to all data in the study and takes responsibility for the integrity of the work and the accuracy of the data analysis.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
There is no personal data shown in this manuscript.
Ethics approval and consent to participate
The ethics committee of the University of Basel approved the initial study protocol, and all patients provided written informed consent for the study, including consent for the use of their data in secondary blood marker analyses and follow-up studies. The ProHOSP trial was registered on 31 July 2006 at http://controlled-trials.com (identifier ISRCTN95122877).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vögeli, A., Ottiger, M., Meier, M.A. et al. Admission levels of asymmetric and symmetric dimethylarginine predict long-term outcome in patients with community-acquired pneumonia. Respir Res 18, 25 (2017). https://doi.org/10.1186/s12931-017-0502-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-017-0502-4