Abstract
Copper indium sulfide (CuInS2) exhibits strong visible light absorption and thus has the potential for good photocatalytic activity; however, rapid charge recombination limits its practical usage. An intriguing strategy to overcome this issue is to couple CuInS2 with another semiconductor to form a heterojunction, which can improve the charge carrier separation and, hence, enhance the photocatalytic activity. In this study, photocatalysts comprising CuInS2 with a secondary CuS phase (termed CuInxSy) and CuInxSy loaded with ZnS (termed ZnS@CuInxSy) were synthesized via a microwave-assisted method. Structural and morphological characterization revealed that the ZnS@CuInxSy photocatalyst comprised tetragonal CuInS2 containing a secondary phase of hexagonal CuS, coupled with hexagonal ZnS. The effective band gap energy of CuInxSy was widened from 2.23 to 2.71 as the ZnS loading increased from 0 to 30%. The coupling of CuInxSy with ZnS leads to long-lived charge carriers and efficient visible-light harvesting properties, which in turn lead to a remarkably high activity for the photocatalytic degradation of brilliant green (95.6% in 5 h) and conversion of 4-nitrophenol to 4-nitrophenolate ions (95.4% in 5 h). The active species involved in these photocatalytic processes were evaluated using suitable trapping agents. Based on the obtained results, photocatalytic mechanisms are proposed that emphasize the importance of h+, O2•–, and OH− in photocatalytic processes using ZnS@CuInxSy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Organic pollutants such as dyes are continuously being released into the environment as a result of increasing agricultural and industrial activities, posing major risks to environmental and public health. Brilliant green (BG) dye is a typical triphenylmethane dye with a complex structure that is stable and non-biodegradable. It is commonly used in biological staining, modern textiles, as well as in the paper, plastic, rubber, cosmetic, and antiseptic industries1. Apart from dyes, 4-nitrophenol (4-NP) is also a common toxic organic contaminant in water. 4-NP exhibits high toxicity and carcinogenicity as well as possessing high chemical and biological stability and water solubility2. As a result, removing organic pollutants including coloured and non-coloured pollutants is a major priority. Many conventional approaches including adsorption have been employed to address organic contaminants. However, there are several drawbacks including low efficiency, limited stability, and high cost3. These disadvantages result in the limited implementation of these techniques on an industrial scale. Semiconductor-based photocatalytic methods have gained substantial attention due to their promising applications in wastewater remediation4,5. Photocatalytic treatment of organic contaminants has the significant benefit of eliminating hazardous pollutants without leaving any harmful products or secondary pollutants6,7,8.
Amongst various semiconductors, CuInS2 is one of the most promising photocatalytic materials due to its narrow band gap energy of ~ 1.45 eV. CuInS2 exists in three different crystal structures including chalcopyrite, zinc blende and wurtzite9. CuInS2 has received increasing attention owing to its long-term stability against photocorrosion, appropriate band gap, and good solar conversion efficiency. It has been reported that it exhibited great potential in H2 evolution from water splitting10,11, photocatalytic degradation of organic pollutants12, photoelectrochemical reduction of CO213, and photocatalytic reduction of Cr(VI)14. A range of synthetic approaches have been used to prepare CuInS2 with different structural compositions and morphology. Various synthetic approaches such as hot injection15, hydrothermal16, solvothermal17, microwave-assisted18, and sonochemical19 methods have been reported. Among these synthesis methods, microwave-assisted synthesis has several advantages in comparison to other more conventional approaches. Microwave assisted synthesis relies on efficient dielectric heating, primarily through two mechanisms, namely dipolar polarization and ionic conduction20. Dipolar polarization occurs when material with partial positive and negative charges aligns with the oscillating microwave field, inducing rotation and generating heat through friction. Ionic conduction involves completely dissolved charged particles oscillating under microwave irradiation leading to collisions with their neighboring molecules/atoms and subsequent heat production21. This method offers short reaction times as well as uniform and rapid heating, resulting in the formation of CuInS2 with high purity and a monodisperse particle size distribution22,23.
Zinc sulfide (ZnS) is another material that is of interest for photocatalytic applications owing to the highly negative reduction potential of photoexcited electrons in the ZnS conduction band in addition to being non-toxic, environmentally friendly, highly stable, and inexpensive24. However, ZnS suffers from some critical drawbacks that limit its photocatalytic efficiency. ZnS is only active under UV light irradiation owing to its wide band gap, which corresponds to just 5% of the total energy available from sunlight. ZnS also suffers from an intrinsically fast electron–hole recombination rate, which competes with interfacial charge transfer and thus limits the photocatalytic efficiency. Therefore, the fabrication of semiconducting heterostructures has become a promising trend and is likely to be the best alternative approach to overcome these drawbacks25. The formation of a heterojunction retains the basic features of the individual components while adding unique multifunctionalities such as enhanced charge separation and transfer of the photogenerated charge carriers between the different semiconductors26,27,28.
Recently, ZnS/CuInS2 heterostructures have drawn a lot of attention, however the usage of this composite in photocatalytic applications has not been fully explored29,30,31. The combination of CuInS2 and ZnS to create a junction shows the advantages of improving the separation and transfer of charge carriers and eventually decreasing the recombination rate of electron–hole pairs. The coupling of CuInS2 with ZnS to form a heterostructure is one of the promising approaches to enhance the photoactivity of both components and extend the photoresponse of the photocatalysts.
To date, the scope of photocatalytic applications of the ZnS@CuInS2 composites has been primarily limited to H2 generation, CO2 reduction, Cr(VI) reduction, and antibiotic and dye degradation31,32,33. However, most previously studied photocatalysts contained other active materials30,34 such as TiO2 and AgInS2 or were modified with mercaptopropionic acid and thioglycolic acid35,36,37.
In this work, CuInS2 with a secondary CuS phase (termed CuInxSy) and CuInxSy loaded with ZnS (termed ZnS@CuInxSy) were prepared via a microwave-assisted method. The effect of different percentages of ZnS on the structural, optical, and morphological properties of the CuInxSy was also studied. Moreover, by loading ZnS onto CuInxSy, the recombination rate of photogenerated carriers was significantly suppressed and an enhancement in the photocatalytic degradation of coloured pollutant, BG, and the conversion of non-coloured pollutant, 4-NP, with a low photocatalyst dosage was achieved. A further in depth study of the active species responsible for the removal of these pollutants was also performed, providing insights into the underlying BG degradation and 4-NP conversion mechanisms. This work demonstrates that the promising ZnS@CuInxSy heterojunction photocatalyst has the potential for the complete elimination of organic pollutants in wastewater.
Experimental section
Chemicals
All reagents were used without further purification. For the synthesis, copper nitrate trihydrate (Cu(NO3)2·3H2O) and indium nitrate (In(NO3)3) were purchased from Alfa Aesar as the Cu and In sources, respectively. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O), thiourea (CH4N2S), hydrogen peroxide (H2O2), and 4-NP (C6H5NO3) were purchased from Merck, Germany. The distilled water was purified using water still from Aquatron, England, and ethanol was purchased from Duksan Pure Chemicals Co. Ltd, South Korea. For the photocatalytic application tests, BG (C27H34N2O4S) was obtained from Sigma-Aldrich. For the trapping experiments, isopropanol and benzoquinone were obtained from Acros, and ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) was purchased from Fluka.
Instrumentation
The CuInxSy and ZnS@CuInxSy composites were synthesized using an Anton-Paar microwave reactor (Monowave 400, Austria). The structure and phase purity of the synthesized materials were analyzed using powder X-ray diffraction (XRD, MiniflexII; Rigaku, Japan). X-ray photoelectron spectroscopy (XPS) was performed using a Kratos Analytical, AXIS Nova to determine the chemical states and elemental compositions of the synthesized materials. Fourier transform infrared spectroscopy (FT-IR) was used to identify the vibrational modes present in the synthesized materials. The FT-IR spectra of the synthesized materials were recorded using an IRspirit Fourier transform infrared spectrometer (IRSpirit, Shimadzu, Japan) in the wavenumber range 400–4000 cm−1 using the KBr pellet method. Raman spectra were obtained using a JASCO NRS-5100 Micro Raman Spectrometer equipped with a 532.06 nm laser. The optical band gap energies of the materials were determined using ultraviolet–visible diffuse reflectance spectroscopy (UV–Vis DRS, Shimadzu UV-2600i, Japan). Further morphological and crystallographic information about the synthesized materials was obtained using field-emission transmission electron microscopy (FE-TEM) and selected area electron diffraction (SAED) conducted with a JEM-F200 (JEOL Ltd., Tokyo, Japan). The average pore size, pore volume, and BET surface area of CuInxSy and 30% ZnS@CuInxSy were measured using a surface area analyzer (Quantachrome autosorb-iQ, Austria). For photocatalytic studies of the synthesized materials, the experiments were carried out in a Toption (TOPT-V) photochemical reactor with a 300 W Xe lamp as the UV–visible light source (wavelength > 350 nm). The intensity of the light at the location of the reaction vessel was ~ 14 mW/cm2, as measured by a Thorlabs PM100D power meter with a S401C thermal sensor. A Shimadzu UV-1900 UV–visible spectrophotometer was utilized to monitor the absorbance of BG and 4-NP in the spectral range 200–800 nm. A general-purpose pH meter (GP353 EDT direction, United Kingdom) was used to determine the pH of the 4-NP aqueous solution.
Synthesis of CuInxSy
CuInxSy was synthesized using a simple microwave-assisted synthesis method as reported in a previous study28. In a typical synthesis, 0.2416 g of Cu(NO3)2·3H2O, 0.3008 g of In(NO3)3, and 0.3045 g of thiourea were added into 15 mL ethylene glycol and the mixture was loaded into a 30 mL quartz vessel (G30 vial). The vessel was purged with N2 gas and sealed with a septum then rapidly heated to 200 °C by 850 W microwave irradiation for 10 min with continuous stirring to ensure homogenous heating. After the resulting product was cooled to ambient temperature, it was washed three times with distilled water and ethanol and collected by centrifugation (3500 rpm, 5 min per wash). Finally, the product was dried at 80 °C for 4 h to yield a black powder. Although pure, stoichiometric CuInS2 was the intended synthesis product, the formation of a CuS secondary phase is almost inevitable; therefore, we use the term CuInxSy throughout the manuscript to simplify the notation.
Synthesis of ZnS@CuInxSy composites
For the synthesis of ZnS@CuInxSy composites containing different weight percentages (%) of ZnS, the procedure is similar to that mentioned above. In brief, a certain amount of Zn(CH3COO)2·H2O and thiourea were added into a suspension of 0.2 g of CuInxSy. The mixtures were purged with N2 gas and heated to 200 °C by 850 W microwave irradiation for 10 min with continuous stirring. The obtained products were washed three times with distilled water and ethanol, dried at 80 °C for 4 h, and labelled as 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy.
Photocatalytic degradation of brilliant green dye
The photocatalytic activity of the as-prepared materials was evaluated by degrading BG dye at room temperature under visible light irradiation. All experiments were carried out in triplicate. A typical process was as follows: 10 mg of synthesized material was dispersed into 50 mL of 10 ppm BG aqueous solution. Then, the suspension was sonicated for 3 min, and it was later kept in the dark for another 3 min with constant stirring to reach an adsorption–desorption equilibrium of BG dye on the surface of the synthesized materials. The suspension was then irradiated with visible light and 3 mL aliquots were collected at intervals of 60 min for 5 h and transferred into centrifuge tubes to separate the photocatalyst from the dye pollutant solution. The aliquots were analyzed using a UV–Vis spectrophotometer in the range 200–800 nm and the photocatalytic activities of the pristine CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy were estimated by measuring the percentage of dye degradation using the following relation:
where A0 denotes the initial absorbance (the absorbance at time t = 0 h) and At denotes the absorbance of the aqueous dye solution after time t of treatment. Both A0 and At are recorded at the absorbance maximum (λmax) of the aqueous BG dye at 620 nm.
Photocatalytic conversion of 4-NP
For the photocatalytic conversion of 4-NP, 10 mg of synthesized material was added and sonicated in 50 mL of 10 ppm 4-NP solution. After 3 min of adsorption/desorption equilibrium, the photocatalytic 4-NP conversion was initiated by irradiating the reaction mixture with a 300 W Xenon lamp. The activity was monitored using a UV–vis spectrophotometer. At fixed intervals of 1 h, 3 mL of aliquots were collected and filtered by centrifugation to separate the photocatalyst before analysis of the solution.
Active species trapping experiments
To further investigate the main reactive species responsible for the photocatalytic degradation of BG using 30% ZnS@CuInxSy composite, trapping experiments were carried out in the presence of three typical trapping agents: isopropanol, benzoquinone, and disodium ethylenediaminetetraacetate (EDTA-2Na), which are utilized as scavengers of •OH, O2•–, and h+, respectively. For 4-NP conversion, H2O2 was used to trap e− in addition to other trapping agents. These trapping agents were added to the aqueous BG dye solution/4-NP solution at the beginning of the photocatalytic reaction. This experiment was carried out under the same conditions used for the BG degradation and 4-NP conversion experiments.
Results and discussions
Powder X-ray diffraction analysis
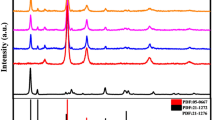
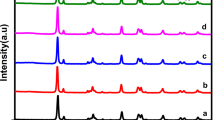
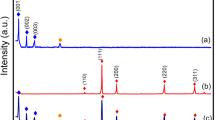
Powder XRD was used to examine the crystal structures and phases of CuInxSy, ZnS, and ZnS@CuInxSy composites with different percentages of ZnS (10%, 20%, and 30%), and the results are displayed in Fig. 1. The XRD pattern of CuInxSy indicated the formation of the tetragonal phase of CuInS2 (ICDD 98–060–0582: indexed in blue) along with the hexagonal phase of CuS (ICDD 98–006–7581: indexed in orange), as shown in Figure S1. This phenomenon suggests that Cu2+ ions could not be completely reduced by ethylene glycol23. The diffraction peaks of ZnS are well-matched with the reported data (ICDD 98–004–2793). The characteristic peaks located at 28.8°, 48.0°, 51.9°, and 56.5° can be observed clearly and corresponds to (002), (110), (103), and (112) of hexagonal ZnS (as indexed in green), respectively38. All of the synthesized ZnS@CuInxSy composites exhibited the main diffraction peaks of CuInS2, CuS, and ZnS. The successful loading of ZnS was confirmed by observation of the broad ZnS diffraction peaks around 28° and 48° superimposed on the CuInS2 and CuS diffraction patterns in the composites, as illustrated in Figure S2. Thus, XRD results confirmed the successful synthesis of ZnS@CuInxSy using microwave-assisted synthesis using ethylene glycol as the solvent.
X-ray photoelectron spectroscopy
The chemical composition and oxidation states of the elements present in CuInxSy and 30% ZnS@CuInxSy were examined by XPS, as shown in Fig. 2. As depicted in the survey XPS (Fig. 2a), peaks corresponding to Cu 2p, In 3d, S 2p, and Zn 2p are observed clearly confirming the successful formation of CuInxSy and ZnS@CuInxSy composites. Figure 2b shows Cu 2p doublets at Cu 2p3/2 (931.8/929.7 eV) and Cu 2p1/2 (951.9/949.6 eV) for CuInxSy and 30% ZnS@CuInxSy, respectively. The results are consistent with the presence of Cu(I), as expected for stoichiometric CuInS2, and in agreement with previous reports32,39. Moreover, the Cu 2p spectra showed no evidence of Cu2+ (in the form of Cu2+ ‘shake-up’ satellites around 944 and 965 eV)40 on the surface of both CuInxSy and 30% ZnS@CuInxSy, which indicates that the CuS secondary phase observed in the XRD patterns is not present on the surface of the photocatalysts. The In 3d XPS spectrum in Fig. 2c exhibited two peaks at 444.1 eV, 451.7 eV for CuInxSy and 442.4 eV, 450.0 eV for 30% ZnS@CuInxSy which correspond to In 3d5/2 and In 3d3/2, respectively. This suggests that the oxidation state of In in the synthesized materials is + 3, as expected for CuInS229. Figure 2d shows that the binding energies of S 2p in CuInxSy are at 162.7 and 161.7 belonging to S 2p1/2 and S 2p3/2, respectively and were separated by a spin–orbit splitting of 1.2 eV. Moreover, the peak positions of S 2p for 30% ZnS@CuInxSy are located at 160.4 and 159.2 eV41. The Zn 2p peaks of ZnS@CuInxSy as shown in Fig. 2e split into Zn 2p3/2 (1019.4 eV) and Zn 2p1/2 (1042.6 eV) can be assigned to Zn2+ with a peak separation of 23.2 eV41. The typical C 1s spectra shown in Fig. 2f arise from adventitious carbon. The XPS results also verify the successful synthesis of ZnS@CuInxSy composite via a microwave-assisted synthesis.
Fourier transform infrared spectroscopy
The vibrational modes present in the synthesized materials were determined via FT-IR. As shown in Fig. 3a, the band located at ~ 500–530 cm−1 can be assigned to the Cu–S42 and In–S43 stretching vibrations. Moreover, no other peaks can be observed in the FT-IR spectra, which reveals that all organic molecules from ethylene glycol and possible byproducts of the synthesis reaction were removed by washing with ethanol and distilled water44,45. For pure ZnS, the bands around 462 cm−1 and 667 cm−1 can be attributed to the characteristic Zn–S stretching vibration modes46,47. While, the bands around 1620 and 2070 cm−1 can be ascribed to the C–N and C=S vibrations of thiourea48. The FT-IR spectra of 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy exhibited similar Cu–S, In–S, and Zn–S bands. This confirms the presence of both CuInxSy and ZnS. Thus, FTIR measurements also verify the successful loading of ZnS onto CuInxSy.
Raman spectroscopy
Raman spectroscopy was employed to study the structure and bonding in CuInxSy, ZnS, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy. Figure 3b shows the Raman scattering spectra of CuInxSy and ZnS@CuInxSy composites with different ZnS contents. The Raman features broaden and shift continuously upward upon increasing ZnS content from 10 to 30%. The Raman spectrum of CuInxSy exhibited a strong peak at 290 cm−1 which may be assigned to the A1 mode of the chalcopyrite CuInS249. A weak peak at 470 cm−1 may be assigned to the S–S stretching vibration mode of hexagonal CuS50,51. Moreover, no detectable peaks were observed for ZnS. Thus, the Raman spectra can only confirm the presence of CuInS2 and a secondary phase of CuS, which is in agreement with the XRD results.
UV–visible diffuse reflectance spectroscopy
The effective band gap energies of the synthesized materials were determined by constructing Tauc plots from Kubelka–Munk transformed diffuse reflectance data, as shown in Fig. 4. The inset of Fig. 4 shows the photographic of synthesized CuInxSy, ZnS, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy. The Kubelka–Munk function was used to estimate the effective band gap energy. The estimated band gap energy of CuInxSy was found to be 2.23 eV. While, for pure ZnS, the Tauc plot reveals a band gap energy of 3.87 eV, which corresponds to the sharp absorption onset at ~ 323 nm. As the ZnS content increases, the band gap energy of CuInxSy gradually increases from 2.23 to 2.51 eV. The effective band gaps of 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy were estimated to be 2.32, 2.41, and 2.51 eV respectively. The ZnS@CuInxSy composites exhibit narrow band gaps, which enable the materials to harvest visible light efficiently.
Transmission electron microscopy
The TEM images of CuInxSy and 30% ZnS@CuInxSy are shown in Fig. 5a and b, respectively. Figure 5a1 and a2 shows the rod-like structure of CuInxSy particles. While 30% ZnS@CuInxSy exhibited a needle-like structure, as shown in Fig. 5b1 and b2, which also shows that ZnS was well dispersed on the surface of the CuInxSy particles, and their close contact resulted in the formation of a ZnS@CuInxSy composite, which is helpful for fast interfacial charge carrier transfer. Thus, the photogenerated charge carriers can be utilized effectively, thereby enhancing the photocatalytic performance.
Figure 6a1 shows the HR-TEM image of CuInxSy, the spacing of the lattice fringe of 0.276 and 0.319 nm matched well with (020) and (112) planes of tetragonal CuInS2, respectively. The 0.319 nm lattice fringe was also observed in Fig. 6b1, the HR-TEM image of ZnS@CuInxSy composites. The additional lattice fringe of 0.192 nm was assigned to the (110) plane of hexagonal ZnS. The SAED patterns of CuInxSy in Fig. 6a2 confirmed the presence of the (112) and (024) planes of tetragonal CuInS2 (indexed in blue) and the (023) plane of hexagonal CuS (indexed in orange). Moreover, the SAED patterns of 30% ZnS@CuInxSy as shown in Fig. 6b2 exhibit bright concentric rings corresponding to the (112) and (224) diffraction planes of tetragonal CuInS2 in addition to the (110) plane of hexagonal ZnS (indexed in green). These results are in accordance with the XRD results, which also confirm the successful loading of ZnS onto CuInxSy.
Brunauer–Emmett–Teller surface area analysis
The BET N2 adsorption–desorption isotherm analysis was carried out to investigate the average pore size, pore volume, and BET surface area of CuInxSy and 30% ZnS@CuInxSy. As shown in Fig. 7, the N2 adsorption/desorption isotherm of CuInxSy and 30% ZnS@CuInxSy revealed a type IV isotherm (according to the IUPAC classification) indicating the mesoporous feature of the materials. From Table 1, the CuInxSy exhibited a surface area of 46.9 m2/g, and with the introduction of 30% ZnS onto CuInxSy, the surface area increased to 55.8 m2/g. The BET analysis revealed that the 30% ZnS@CuInxSy has a surface area with pore size and pore volume of approximately 142.7 Å and 0.398 cm3/g, respectively. This suggests that the addition of ZnS could provide more active surface sites. Thus, providing enough active sites for the adsorption of dyes on the surface of the photocatalyst and photocatalysis to take place.
Photocatalytic activity of ZnS@CuInxSy
Photocatalytic degradation of brilliant green
The photocatalytic efficiencies of CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy for BG dye are presented in Fig. 8a. Prior to light irradiation, the dye and photocatalyst suspension were kept in the dark for 3 min under constant stirring to achieve an adsorption–desorption equilibrium. The percentages of BG adsorption were found to be 41.5% ± 3.96%, 53.7% ± 2.93%, 51.4% ± 1.05%, and 60.7% ± 2.06% for CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy, respectively, after 5 h in the dark, as shown in Figure S3. This shows that the CuInxSy and ZnS@CuInxSy composites have a high adsorptive affinity towards BG dye, which is crucial because if the dye molecules cannot be adsorbed on the surface of the synthesized materials, the photocatalytic activity would not be effective52. This may also be associated with the larger surface area, pore volume, and pore size of 30% ZnS@CuInxSy compared with CuInxSy, as shown in Table 1. During the course of the photocatalytic reaction, the intensity of the BG dye solution gradually diminishes, and a gradual decrease in the absorption spectra of BG can be observed at the characteristic absorption peak height around 620 nm. Moreover, unmodified CuInxSy exhibited a poor photocatalytic performance of about 44.5% ± 1.36% under irradiation by visible light, which may be ascribed to the rapid recombination of charge carriers. The photocatalytic performance of CuInxSy is significantly enhanced as the amount of ZnS loaded increases from 10 to 30%. The photocatalytic activity of 30% ZnS@CuInxSy against BG was found to be considerably higher than those of unmodified CuInxSy, 10% ZnS@CuInxSy, and 20% ZnS@CuInxSy. Within 5 h, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy were able to degrade about 79.2% ± 1.66%, 92.0% ± 0.50%, and 95.6% ± 0.08% of BG, respectively under visible light irradiation. The improved photocatalytic performance of 30% ZnS@CuInxSy is likely due to the presence of ZnS on the surface of CuInxSy, which facilitates the separation and transfer of the photogenerated electrons and holes effectively resulting in superior degradation efficiency.
(a) Percentage photocatalytic degradation of BG using CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy under visible light irradiation and (b) photocatalytic activity of 30% ZnS@CuInxSy with different trapping agents to determine the main reactive species responsible for the photocatalytic degradation of BG.
Three different scavengers including benzoquinone for O2•–, EDTA for h+, and isopropanol for •OH were used to evaluate the main active species involved in the photocatalytic degradation of BG. As shown in Fig. 8b, the photocatalytic degradation of BG hardly decreased with the addition of isopropanol in comparison to other scavengers. Moreover, when benzoquinone was used to scavenge O2•–, a notable decrease in photocatalytic efficiency of 30% ZnS@CuInxSy. The addition of EDTA showed the highest inhibition of the photocatalytic degradation of 30% ZnS@CuInxSy which confirmed the influence of h+ in the photocatalytic process.
Photocatalytic conversion of 4-NP
The photocatalytic conversion of 4-NP to the 4-nitrophenolate ion by CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy, as evidenced by UV–Vis absorbance spectroscopy, is shown in Fig. 9. In both the absence and presence of light, CuInxSy exhibited poor photocatalytic conversion activity in comparison to the other synthesized materials, as shown in Fig. 9a and b. Interestingly, when the ZnS@CuInxSy composites were added and irradiated with visible light, the maximum absorption peak of 4-NP shifted from 316 to 400 nm, and the colour of the solution changed from colourless to pale yellow due to the deprotonation of 4-NP to form the 4-nitrophenolate ion53. The conversion of 4-NP was gradually improved as the amount of ZnS loaded on the surface of CuInxSy increased from 10 to 30%. Among the synthesized materials, 30% ZnS@CuInxSy showed the highest conversion under irradiation by visible light. The active species involved in the process of 4-NP conversion were further identified by adding BQ, EDTA, isopropanol, and H2O2 to the solution mixture as scavengers to capture O2•–, h+, •OH, and e−, respectively. Based on the reactive species trapping experiments (Fig. 9c), the addition of benzoquinone, isopropanol, and H2O2 resulted in equal inhibition of 4-NP conversion, which indicates that O2•–, •OH, and e− play equal roles in the conversion process. Moreover, the addition of EDTA to the mixture exhibited the highest inhibition of 4-NP conversion, which implies that photogenerated h+ are the main active species involved in the conversion process.
Photocatalytic conversion of 4-NP using CuInxSy, 10% ZnS@CuInxSy, 20% ZnS@CuInxSy, and 30% ZnS@CuInxSy (a) in the dark, (b) under visible light irradiation, and (c) photocatalytic activity of 30% ZnS@CuInxSy with different trapping agents to determine the main reactive species responsible for the photocatalytic conversion of 4-NP.
Proposed photocatalytic mechanism
The proposed photocatalytic mechanism of BG degradation using ZnS@CuInxSy is illustrated in Fig. 10 based on the band gap energy, the reported energies (specified as potentials vs. NHE) of the conduction band (CB) and valence band (VB) of ZnS and CuInS2, and the trapping experiments. The secondary CuS phase is not separately considered here because the XPS results indicate that it is not present at the surface of the photocatalyst particles. Based on the literature, CuInxSy is assumed to have ECB = − 0.34 V and EVB = 1.23 V14, while ZnS has ECB = − 1.56 V and EVB = 3.06 V54. Upon visible light irradiation, ZnS and CuInxSy simultaneously generate e− and h+, in which photogenerated e− are excited to their CB, leaving behind h+ in the VB. Next, the photogenerated e− in the CB of ZnS are transferred to the VB of CuInxSy, which improves the separation of photogenerated charge carriers and prolongs their lifetime. This also increases the probability of the photogenerated charge carriers participating in the redox reaction on the surface, which effectively improves the photocatalytic degradation performance. The photogenerated e− in the CB of CuInxSy (ECB = − 0.34 V) can reduce dissolved O2 to yield O2•– (O2/O2•–, − 0.33 V vs. NHE), which agrees with the results of the active species trapping experiments. Alternatively, the photogenerated e− can reduce the BG dye directly. Moreover, the photogenerated h+ of ZnS can react with H2O or OH− species, oxidizing them into •OH radicals, or the h+ can directly oxidize the BG dye to CO2 and H2O, which are harmless end products. Based on the active species trapping study, EDTA showed the highest inhibition followed by benzoquinone when compared to no scavenger, which indicates that h+ and O2•– play important roles in the photocatalytic degradation process. Moreover, isopropanol shows the lowest inhibition, implying that •OH is the least important species in the photocatalytic degradation of BG by ZnS@CuInxSy.
In the case of the photocatalytic conversion of 4-NP, the enhanced conversion may be ascribed to the presence of OH−, which can either be formed through (i) direct reduction of H2O by e−cb, (ii) reduction of O2 to O2•– and subsequent reaction with H2O to form H2O2 and OH−, or (iii) a two-step process in which H2O is first oxidized to •OH by h+vb and then e−cb reduces •OH to yield OH− ions. It must be noted that in order for these reactions to result in a net increase in pH, some h+ must be consumed in a process other than water oxidation and/or H+ must be consumed by another process (e.g., conversion of 4-NP and/or 4-nitrophenolate ion to hydroquinone, as evidenced by the peak located ~ 225 nm in Fig. 9)55. The presence of OH− increases the pH of the solution from 7.00 to slightly basic ~ 7.22, which deprotonates 4-NP to the 4-nitrophenolate ion. It is worth noting that the significance of the quite small pH change is dependent on the pKa of 4-NP. Since the pKa of 4-NP is 7.1556, this pH change is expected to cause a shift in the acid–base equilibrium from most of the 4-NP being protonated ([A–]/[HA] ≈ 0.71, according to the Henderson-Hasselbalch equation) to most being deprotonated ([A–]/[HA] ≈ 1.17), which is consistent with the observed changes in absorbance57.
Conclusion
A series of highly efficient ZnS@CuInxSy nanocomposite photocatalysts with different ZnS loadings have been successfully synthesized via a microwave-assisted method and applied for the photocatalytic degradation of BG dye and 4-NP in aqueous solution. When compared to unmodified CuInxSy, the composite materials showed enhanced photocatalytic performance under visible light irradiation, particularly 30% ZnS@CuInxSy. Active species trapping experiments indicate that mainly h+ and O2•– are involved in the photocatalytic processes. The formation of a heterojunction between CuInxSy and ZnS decreases photogenerated charge carrier recombination and thereby enhances the carrier separation efficiency in the composite. Therefore, this study provides a simple and effective route for the synthesis of a new visible-light active photocatalyst material and also highlights the importance of its application in the elimination of different organic contaminants from wastewater.
Data availability
All data generated or analyzed during this study are included in the manuscript and SI.
References
Singh, S., Gupta, H., Dhiman, S. & Sahu, N. K. Decontamination of cationic dye brilliant green from the aqueous media. Appl. Water Sci. 12(4), 61. https://doi.org/10.1007/s13201-022-01596-5 (2022).
Li, Q., Qiao, X.-Q., Jia, Y., Hou, D. & Li, D.-S. Amorphous CoMoS4 nanostructure for photocatalytic H2 generation, nitrophenol reduction, and methylene blue adsorption. ACS Appl. Nano Mater. 3(1), 68–76. https://doi.org/10.1021/acsanm.9b01582 (2020).
Pillai, S. B. Adsorption in Water and Used Water Purification. In Handbook of Water and Used Water Purification 1–22 (Springer International Publishing, 2020). https://doi.org/10.1007/978-3-319-66382-1_4-1.
Khan, M. M. Chalcogenide-Based Nanomaterials as Photocatalysts (Elsevier, 2021). https://doi.org/10.1016/C2019-0-01819-5.
Khan, M. M. Principles and Mechanisms of Photocatalysis. In Photocatalytic Systems by Design 1–22 (Elsevier, 2021). https://doi.org/10.1016/B978-0-12-820532-7.00008-4.
Khan, M. M. Theoretical Concepts of Photocatalysis (Elsevier, 2023). https://doi.org/10.1016/C2021-0-01798-3.
Rahman, A. & Khan, M. M. Chalcogenides as photocatalysts. New J. Chem. 45, 19622–19635. https://doi.org/10.1039/d1nj04346c (2021).
Khan, M. M., Pradhan, D. & Sohn, Y. Nanocomposites for Visible Light-Induced Photocatalysis. In Springer Series on Polymer and Composite Materials (eds Khan, M. M. et al.) (Springer International Publishing, 2017). https://doi.org/10.1007/978-3-319-62446-4.
Rahman, A., Jennings, J. R. & Khan, M. M. CuInS2 and CuInS2-based nanostructures as photocatalysts. Mater. Sci. Semicond. Process. 169, 107930. https://doi.org/10.1016/j.mssp.2023.107930 (2024).
Cai, Q., Liu, Z., Han, C., Tong, Z. & Ma, C. CuInS2/Sb2S3 heterostructure modified with noble metal co-catalyst for efficient photoelectrochemical water splitting. J. Alloys Compd. 795, 319–326. https://doi.org/10.1016/j.jallcom.2019.04.312 (2019).
Khan, M. M. & Rahman, A. Chalcogenides and chalcogenide-based heterostructures as photocatalysts for water splitting. Catalysts 12(11), 1338. https://doi.org/10.3390/catal12111338 (2022).
Kang, S.-Z., Yang, Y.-K., Bu, W. & Mu, J. TiO2 nanoparticles incorporated with CuInS2 clusters: Preparation and photocatalytic activity for degradation of 4-nitrophenol. J. Solid State Chem. 182(11), 2972–2976. https://doi.org/10.1016/j.jssc.2009.08.014 (2009).
Toyodome, T., Amao, Y. & Higashi, M. Photoelectrochemical reduction of CO2 to formate over a hybrid system of CuInS2 photocathode and formate dehydrogenase under visible-light irradiation. New J. Chem. 45(32), 14803–14807. https://doi.org/10.1039/D1NJ02481G (2021).
Deng, F. et al. Novel visible-light-driven direct Z-scheme CdS/CuInS2 nanoplates for excellent photocatalytic degradation performance and highly-efficient Cr(VI) reduction. Chem. Eng. J. 361, 1451–1461. https://doi.org/10.1016/j.cej.2018.10.176 (2019).
Perner, V. et al. Hot injection synthesis of CuInS2 nanocrystals using metal xanthates and their application in hybrid solar cells. New J. Chem. 43(1), 356–363. https://doi.org/10.1039/C8NJ04823A (2019).
Raja, A., Son, N., Swaminathan, M. & Kang, M. Facile synthesis of sphere-like structured ZnIn2S4-RGO-CuInS2 ternary heterojunction catalyst for efficient visible-active photocatalytic hydrogen evolution. J. Colloid Interface Sci. 602, 669–679. https://doi.org/10.1016/j.jcis.2021.06.034 (2021).
Huang, W.-C. et al. Solvothermal synthesis of zincblende and wurtzite CuInS2 nanocrystals and their photovoltaic application. Langmuir 28(22), 8496–8501. https://doi.org/10.1021/la300742p (2012).
Chumha, N. et al. Photocatalytic activity of CuInS2 nanoparticles synthesized via a simple and rapid microwave heating process. Mater. Res. Express 7(1), 015074. https://doi.org/10.1088/2053-1591/ab6885 (2020).
Amiri, O. et al. A new sonochemical method for preparation of different morphologies of CuInS2 nanostructures. Bull. Mater. Sci. 37(5), 1079–1085. https://doi.org/10.1007/s12034-014-0047-9 (2014).
Mingos, D. M. P. & Baghurst, D. R. Tilden lecture. Applications of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 20(1), 1–47. https://doi.org/10.1039/cs9912000001 (1991).
Krishnan, R. et al. Recent advances in microwave synthesis for photoluminescence and photocatalysis. Mater. Today Commun. 32, 103890. https://doi.org/10.1016/j.mtcomm.2022.103890 (2022).
Mange, Y. J., Dewi, M. R., Macdonald, T. J., Skinner, W. M. & Nann, T. Rapid microwave assisted synthesis of nearly monodisperse aqueous CuInS2/ZnS nanocrystals. CrystEngComm 17(41), 7820–7823. https://doi.org/10.1039/C5CE01325A (2015).
Hosseinpour-Mashkani, S. M., Salavati-Niasari, M., Mohandes, F. & Venkateswara-Rao, K. CuInS2 nanoparticles: Microwave-assisted synthesis, characterization, and photovoltaic measurements. Mater. Sci. Semicond. Process. 16(2), 390–402. https://doi.org/10.1016/j.mssp.2012.09.005 (2013).
Jiang, F. et al. Visible light photocatalytic H2-production activity of epitaxial Cu2ZnSnS4/ZnS heterojunction. Catal. Commun. 85, 39–43. https://doi.org/10.1016/j.catcom.2016.07.017 (2016).
Rahman, A., Jennings, J. R., Tan, A. L. & Khan, M. M. Molybdenum disulfide-based nanomaterials for visible-light-induced photocatalysis. ACS Omega 7(6), 22089–22110. https://doi.org/10.1021/acsomega.2c01314 (2022).
Amaranatha Reddy, D., Ma, R., Choi, M. Y. & Kim, T. K. Reduced graphene oxide wrapped ZnS–Ag2S ternary composites synthesized via hydrothermal method: Applications in photocatalyst degradation of organic pollutants. Appl. Surf. Sci. 324, 725–735. https://doi.org/10.1016/j.apsusc.2014.11.026 (2015).
Zhang, Y. et al. Facile preparation of high-performance hydrochar/TiO2 heterojunction visible light photocatalyst for treating Cr(VI)-polluted water. Colloids Surf. A Physicochem. Eng. Asp. 681, 132775. https://doi.org/10.1016/j.colsurfa.2023.132775 (2024).
Rahman, A., Khan, F., Jennings, J. R., Kim, Y.-M. & Khan, M. M. CdS@CuInS2 nanocomposites for enhanced photocatalytic activity under visible light irradiation. Mater. Sci. Semicond. Process. 177, 108365. https://doi.org/10.1016/j.mssp.2024.108365 (2024).
Yan, L., Li, Z., Sun, M., Shen, G. & Li, L. Stable and flexible CuInS2/ZnS:Al-TiO2 film for solar-light-driven photodegradation of soil fumigant. ACS Appl. Mater. Interfaces 8(31), 20048–20056. https://doi.org/10.1021/acsami.6b05587 (2016).
Dai, Y.-W. et al. Au@SiO2@CuInS2–ZnS/Anti-AFP fluorescent probe improves HCC cell labeling. Hepatobiliary Pancreat. Dis. Int. 18(3), 266–272. https://doi.org/10.1016/j.hbpd.2019.03.001 (2019).
Tsuji, I., Kato, H. & Kudo, A. Photocatalytic hydrogen evolution on ZnS−CuInS2−AgInS2 solid solution photocatalysts with wide visible light absorption bands. Chem. Mater. 18(7), 1969–1975. https://doi.org/10.1021/cm0527017 (2006).
Cui, Q., Gu, X., Zhao, Y., Qi, K. & Yan, Y. S-scheme CuInS3/ZnS heterojunctions for the visible light-driven photocatalytic degradation of tetracycline antibiotic drugs. J. Taiwan Inst. Chem. Eng. 142, 104679. https://doi.org/10.1016/j.jtice.2023.104679 (2023).
Sandroni, M. et al. Cadmium-free CuInS2/ZnS quantum dots as efficient and robust photosensitizers in combination with a molecular catalyst for visible light-driven H2 production in water. Energy Environ. Sci. 11(7), 1752–1761. https://doi.org/10.1039/C8EE00120K (2018).
Ren, Y.-Y., Xia, W., Deng, B.-Y., Liu, J. & Wang, F. Host-guest assemblies of anchoring molecular catalysts of CO2 reduction onto CuInS2/ZnS quantum dots for robust photocatalytic syngas production in water. Mol. Catal. 520, 112168. https://doi.org/10.1016/j.mcat.2022.112168 (2022).
Kong, L., Li, Z., Huang, S., Jia, J. & Li, L. Boosting photocatalytic performance and stability of CuInS2/ZnS–TiO2 heterostructures via sol-gel processed integrate amorphous titania gel. Appl. Catal. B 204, 403–410. https://doi.org/10.1016/j.apcatb.2016.11.055 (2017).
Mohamed, N. B. H. et al. Statistical physics analysis of adsorption isotherms and photocatalysis activity of MPA coated CuInS2/ZnS nanocrystals for the removal of methyl blue from wastewaters. Inorg. Chem. Commun. 144, 109933. https://doi.org/10.1016/j.inoche.2022.109933 (2022).
Zhang, Z. et al. CuInS2/ZnS/TGA nanocomposite photocatalysts: Synthesis characterization and photocatalytic activity. Catal. Lett. 147(7), 1631–1639. https://doi.org/10.1007/s10562-017-2067-8 (2017).
Zhao, Y., Zhang, Y., Zhu, H., Hadjipanayis, G. C. & Xiao, J. Q. Low-temperature synthesis of hexagonal (wurtzite) ZnS nanocrystals. J. Am. Chem. Soc. 126(22), 6874–6875. https://doi.org/10.1021/ja048650g (2004).
Li, R. et al. Development of a fluorometric and colorimetric dual-mode sensing platform for acid phosphatase assay based on Fe3+functionalized CuInS2/ZnS quantum dots. Anal. Chim. Acta https://doi.org/10.1016/j.aca.2023.342121 (2023).
Xie, X. et al. Synthesis of a Cu2-xSe/g-C3N4 heterojunction photocatalyst for efficient photocatalytic H2 evolution. Colloids Surf. A Physicochem. Eng. Asp. 635, 128103. https://doi.org/10.1016/j.colsurfa.2021.128103 (2022).
Chetty, S. S., Praneetha, S., Vadivel Murugan, A., Govarthanan, K. & Verma, R. S. Microwave-assisted synthesis of quasi-pyramidal CuInS2–ZnS nanocrystals for enhanced near-infrared targeted fluorescent imaging of subcutaneous melanoma. Adv. Biosyst. 3(1), 1800127. https://doi.org/10.1002/adbi.201800127 (2019).
Hemathangam, S., Thanapathy, G. & Muthukumaran, S. Tuning of band gap and photoluminescence properties of Zn doped Cu2S thin films by CBD method. J. Mater. Sci. Mater. Electron. 27(2), 2042–2048. https://doi.org/10.1007/s10854-015-3989-9 (2016).
Timoumi, A. et al. Impact of thermal annealing inducing oxidation process on the crystalline powder of In2S3. J. Mater. Sci. Mater. Electron. 31(16), 13636–13645. https://doi.org/10.1007/s10854-020-03921-7 (2020).
Fakhri, H., Mahjoub, A. R. & Khavar, A. H. C. Synthesis and characterization of ZnO/CuInS2 nanocomposite and investigation of their photocatalytic properties under visible light irradiation. Appl. Surf. Sci. 318, 65–73. https://doi.org/10.1016/j.apsusc.2014.01.024 (2014).
Mousavi-Kamazani, M., Salavati-Niasari, M. & Emadi, H. Synthesis and characterization of CuInS2 nanostructure by ultrasonic-assisted method and different precursors. Mater. Res. Bull. 47(12), 3983–3990. https://doi.org/10.1016/j.materresbull.2012.08.044 (2012).
Besra, S. et al. Facile synthesis and characterization of ZnO:Al/ZnS/NiO heterojunction thin films with enhanced photocatalytic activities. Solid State Sci. 143, 107282. https://doi.org/10.1016/j.solidstatesciences.2023.107282 (2023).
Zafar, S. et al. Effect of Fe doping on the structural and optical properties of ZnS macro-spheres. Optik (Stuttg) 262, 169342. https://doi.org/10.1016/j.ijleo.2022.169342 (2022).
Faryal, S. et al. Thiourea-capped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules 27(18), 5744. https://doi.org/10.3390/molecules27185744 (2022).
Yue, W. et al. l-cysteine assisted-synthesis of 3D In2S3 for 3D CuInS2 and its application in hybrid solar cells. RSC Adv. 7(60), 37578–37587. https://doi.org/10.1039/C7RA05730J (2017).
Rajaram, R. et al. Amperometric determination of Myo-inositol using a glassy carbon electrode modified with nanostructured copper sulfide. Microchim. Acta 187(6), 334. https://doi.org/10.1007/s00604-020-04300-z (2020).
Zhang, L. et al. Annealing effects on CuInS2 thin films grown on glass substrates by using pulsed laser deposition. J. Korean Phys. Soc. 64(3), 410–414. https://doi.org/10.3938/jkps.64.410 (2014).
Michalec, K. & Kusior, A. From adsorbent to photocatalyst: The sensitization effect of SnO2 surface towards dye photodecomposition. Molecules 26(23), 7123. https://doi.org/10.3390/molecules26237123 (2021).
Zhang, J. et al. Synthesis of an Ag@AgCl catalyst with amorphous copper as the support and its catalytic performance in the reduction of 4-nitrophenol. J. Chem. Res. 45(5–6), 551–557. https://doi.org/10.1177/1747519820942018 (2021).
Isac, L. & Enesca, A. Recent developments in ZnS-based nanostructures photocatalysts for wastewater treatment. Int. J. Mol. Sci. 23(24), 15668. https://doi.org/10.3390/ijms232415668 (2022).
Wilke, T., Schneider, M. & Kleinermanns, K. 1,4-hydroquinone is a hydrogen reservoir for fuel cells and recyclable via photocatalytic water splitting. Open J. Phys. Chem. 03(02), 97–102. https://doi.org/10.4236/ojpc.2013.32012 (2013).
Pintar, A. & Levec, J. Catalytic oxidation of aqueous p-chlorophenol and p-nitrophenol solutions. Chem. Eng. Sci. 49(24), 4391–4407. https://doi.org/10.1016/S0009-2509(05)80029-6 (1994).
Bhagavan, N. V. Water, Acids, Bases, and Buffers. In Medical Biochemistry 1–16 (Elsevier, 2002). https://doi.org/10.1016/B978-012095440-7/50003-2.
Acknowledgements
This research was funded by Universiti Brunei Darussalam through grants UBD/RSCH/1.4/FICBF(b)/2021/035 and UBD/RSCH/1.4/FICBF(b)/2023/059, and by the Basic Science Research Program through the National Research Foundation (NRF) of Korea grant funded by the Ministry of Education (2021R1A6A1A03039211 and 2022R1A2B5B01001998).
Author information
Authors and Affiliations
Contributions
A.R: Methodology, investigation, data curation, writing—original draft. F.K.: Formal analysis and data curation. J.R.J.: Supervision, writing, review & editing. Y.-M.K.: Resources, funding, and formal analysis. M.M.K.: Supervision, conceptualization, funding acquisition, writing, review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, A., Khan, F., Jennings, J.R. et al. Microwave-assisted synthesis of ZnS@CuInxSy for photocatalytic degradation of coloured and non-coloured pollutants. Sci Rep 14, 16155 (2024). https://doi.org/10.1038/s41598-024-66100-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66100-2
- Springer Nature Limited