Abstract
Brilliant green is a synthetic and toxic dye that is currently being utilized for various purposes, such as dying paper, leather, wool, and silk. The present study demonstrates the activated carbon preparation from waste banana peels as well as its usefulness to remove cationic dye brilliant green from aqueous medium. The dye removal was examined under a set of diverse conditions. The obtained results indicate that dye adsorption was maximum after 60 min of contact time. The removal of brilliant green dye enhances due to a rise in adsorbent dosage and becomes quantitative at 15 min of adsorbent dose. At a pH of 2, the uptake of dye by adsorbent was maximum, which decreases with the rise in pH. Langmuir isotherm was slightly better fitted than Freundlich model at varying temperatures. The experimental value of adsorption capacity was > 900 mg/g, which was observed quite close with pseudo-second-order model for brilliant green adsorption on the prepared adsorbent based on banana peel. Thermodynamic studies suggested exothermic, spontaneous, and favorable adsorption process for brilliant green dye. The adsorbent prepared in the present study can be incorporated for the treatment of wastewater contaminated with brilliant green as well as other toxic pollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the modern era, dyes have substantially ravaged aquatic environments and human health. The worldwide industrialization has enhanced pressure on scientific community engaged in terrestrial as well as wastewater treatment (Kumar et al. 2021a, b; Gupta and Kumar 2020). The major visible concern indicating the unfitness of water for drinking is coloration in water and due to the presence of dyes and pigments. Based on the available literature, around one lakh different kinds of dyes are produced at a production rate of 7 × 105, annually (Vasuki et al. 2017; Bahl et al. 2007; Pathania et al. 2017). Dyes are associated with numerous industries, such as cosmetics, fiber, textile, glass, ceramics, and pharmaceutical industries (Agarwal et al. 2017; Ghaedi et al. 2015). Industries that generate colored effluents containing dye and pigments discharge the same to the natural water bodies. Various studies have been reported in literature showing various contaminants in fresh water system (Kumar et al. 2015, 2017). The presence of effluents in water bodies affects the aquatic biota and also humans by fluctuating pH with large suspended solids having high oxygen demand. These also reduce light penetration and visibility (Bhattacharyya and Sarma 2003; Sawant et al. 2017). Treatment of dye effluent is a big issue as it contains aromatic molecular structure due to which it becomes more stable and more difficult to biodegrade (Vasuki et al. 2017). The assessment of risk towards human health and eco-friendly environment is essential (Kumar et al. 2021c). One of the toxic dyes is brilliant green (BG) which causes several lungs, skin and many other respiratory problems that have been reported globally (Ali et al. 2017). Brilliant green dye causes serious injuries if comes directly in contact with eye. Brilliant green is a synthetic and toxic dye that is currently being utilized for various purposes, such as dying paper, leather, wool, and silk. It is also applied in detection and determination of metals. Various industries employed biological treatment, coagulation, floatation, bacterial degradation, oxidation, hyperfiltration, and adsorption to remove such toxic dye from the water bodies (Bhattacharyya et al. 2017; Agarwal et al. 2017; Sawant et al. 2017). Recently, sequestration of carbon from forest soils has also been reported (Kumar et al. 2021d). Among these methods, the advantage of adsorption is versatility, easy operation, and the application of low-cost materials. Furthermore, the advantage of activated carbon is high carbon and low organic content. A few of the low-cost materials employed for adsorption are agricultural/fertilizer wastes or by-products, such as rice, husk, coffee, bean husk, crushed fruit wastes, such as banana pith, orange peel sugar cane bagasse, shale oil ash (Sawant et al. 2017; Annadurai et al. 2002; Namasivayam and Kavitha 2002; Mane et al. 2007; Tavlieva et al. 2013).
The present study deals with finely ground banana peel powder after activation as activated carbon for fast adsorption and removal of hazardous brilliant green dye from the aqueous solution. The banana peel activated carbon (BPAC) was prepared via H3PO4-assisted chemical activation. The various experimental parameters were investigated and adsorption isotherms were applied. Different thermodynamic and kinetic studies were carried out to investigate the feasibility of prepared activated carbon towards adsorption of brilliant green dye.
Materials and methods
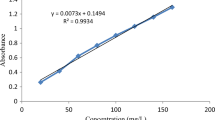
Brilliant green (4-((4-(diethyl amino)-alpha-phenyl benzylidene)-2,5-cyclohexadien-1-ylidene) diethyl ammonium sulfate), a synthetic dye which exists in cationic form with molecular formula C27H34N2O4S and molecular weight 482.63 g mol−1 was purchased from RANKEM. The obtained brilliant green dye was of analytical grade and used as such without any purification. The raw banana peels were collected from a local market in Moradabad. Activated carbon (BPAC) was prepared from raw banana peels using the previously described method (Gupta and Gupta 2016). The schematic representation of activated carbon preparation is given in Fig. 1. The stock solution of 1000 mg/L was obtained using an accurately measured quantity (0.1 g) of dye in solid phase. The experimental solution of concentrations desired is prepared by stock solution through successive dilution. UV–Visible spectrophotometer was utilized for the determination of dye concentration.
Adsorption studies
In a volumetric flask of 100 mL, 30 mL of the dye solution of brilliant green of known concentration was taken. Adsorption study of BG onto BPAC was carried out in batch mode at ambient temperature and definite pH. The solution pH was maintained by NaOH (0.1 N) and HCl (0.1 N) solutions. Desired size of the adsorbent materials was taken and studies were carried out by changing the adsorbent dosage (BPAC varying from 1 to 20 mg), concentration of adsorbate (10–150 mg/L), pH of solution (pH 2–12), contact time (10–90 min). To observe the effect of temperature on dye adsorption, experiments are carried out at different temperatures, i.e. 30–70 °C. After the desired time, the solutions were filtered with Whatman filter paper and the amount of the dye uptake was analyzed spectrophotochemically at λmax 623 nm.
Adsorption kinetics
The brilliant green adsorption on BPAC surface was examined by kinetic models to investigate the adsorption process in the present study. The removal of BG on BPAC was analysed by the most applicable models, namely pseudo-first-order model and pseudo-second-order model (Nandi et al. 2009).
The pseudo-first-order model is represented by the following equation:
where k1 (min−1) represents pseudo-first-order rate constant, qe, and qt represents adsorbed dye amount at equilibrium and at contact time t (min). After integrating the equation within the boundary condition as initial time (t = 0) and at any time t (t > 0), therefore, the rate law for a pseudo-first-order-kinetic reaction becomes:
The sorption kinetics by pseudo-second-order model may be represented as follows:
where k2 represents the constant of pseudo-second-order adsorption (gmg−1 min−1). After integration and using boundary condition as t = 0; qt = 0 and at t = t, adsorbed dye amount was qt. After rearranging, the pseudo-second-order model can be expressed as:
Adsorption isotherm
Adsorption isotherm expresses the relationship between adsorbate per unit weight of adsorbed amount and adsorbate concentration in bulk solution during adsorption at a certain temperature (Kong et al. 2016). It is a useful method that provides information regarding the efficiency of adsorbent to remove the adsorbate. The two most commonly applied models for adsorption isotherm are Langmuir and Freundlich isotherm.
Langmuir isotherm is the best known of all isotherms describing sorption process at a specific homogeneous adsorbent surface (Langmuir 1918; Agarwal et al. 2017). The Langmuir isotherm was represented by the following equation:
where Ce (mg L−1) represents equilibrium BG concentration, qm represents monolayer adsorption capacity (mg g−1), b is Langmuir constant (L mg−1) related to apparent energy of adsorption.
Heterogeneous sorption was explained by Freundlich isotherm. The adsorption of inorganic and organic components in solution is also described by Freundlich isotherm (Agarwal et al. 2017; Ghasemi et al. 2014).
The Freundlich equation is an empirical equation that calculates the isotherm constant represented by the following equation:
Kf represents the Freundlich constant, 1/n is the adsorption intensity and determined by the graph of the qe and Ce. When the value of n is greater than 1 (n > 1), it indicates that the sorption is in favorable conditions.
Thermodynamic parameters
The adsorption process nature and spontaneity are analysed by various thermodynamic parameters, namely Gibbs energy (∆G0), entropy of adsorption (∆S0), and enthalpy of adsorption (∆H0). The different parameters were calculated at different temperatures using the equations:
Ka refers to Langmuir equilibrium constant, T refers to absolute temperature and R represents gas constant.
Results and discussion
Influence of contact time
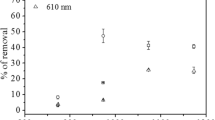
The percentage adsorption of brilliant green (BG) on banana peel activated carbon (BPAC) steadily increases with rise in time of contact and showed constant percentage adsorption at 70 min contact time. The maximum dye removal occurs during initial phase due to the availability of maximum sites which are active for dye sorption and as time increases the dye removal enhances slowly due to the lesser number of active sites left for adsorption (Jain et al. 2018). Figure 2a shows that the dye adsorption in maximum amount occurs within 60 min and becomes gradual thereafter. After 60 min, equilibrium in brilliant green dye adsorption was observed.
Influence of adsorbent dosage
Figure 2b shows the extent of removal of BG dye with the change in adsorbent dosage. Adsorbent surface area and availability of more adsorption sites play a key role in the increase in adsorption with the adsorbent dosage. As adsorbent pore size and vacant sites match with the adsorbate the dye molecule adsorption amount to the adsorbent surface increases otherwise it limits the rate of adsorption (Agarwal et al. 2017). The tendency for adsorption of dye molecule is very high due to high surface area and small particle size of activated carbon. The removal percent adsorption increases up to > 99% as the amount of adsorbent increased from 1 to 15 mg and thereafter becomes constant. The major adsorption of brilliant green dye on BPAC may occur due to Van der Waals attractive forces, π–π interaction, and hydrogen bonding. Furthermore, with the increase in surface area, the number of active sites is increased due to which the adsorption percentage of brilliant green dye onto BPAC is increased. A similar observation was reported by Ghaedi et al. (2014) for the removal of methylene blue dye onto activated carbon developed from peanut stick. Mall et al. (2005) also reported the increase in adsorption with an increase in adsorbent dose for the removal of malachite green dye onto bagasse fly ash, commercial and laboratory activated carbons. Agarwal et al. (2017) observed maximum adsorption of 94.80% brilliant green dye onto the adsorbent prepared from Peganum harmala-L seeds.
Effect of pH
The effect of pH on the dye for powdered activated carbon was observed in the pH range 2–12. The structural stability and color intensity of BG molecule were pH-dependent (Rehman et al. 2011; Mane et al. 2007). The surface charge as well as the degree of ionization of the adsorbent in the solution is highly affected by the pH parameter. The maximum % age removal of dye was obtained when the pH was 2.0. At a pH of 2.0, the percentage removal of BG using BPAC was 97.4% (Fig. 2c). The adsorption rate was found to decrease with increase in pH. The adsorbent BPAC also showed maximum adsorption at pH 2.0 for anthracene removal from aqueous medium (Gupta and Kumar, 2016). Rehman et al. (2011) also reported the maximum adsorption of brilliant green dye in acidic medium on radish peels as well as on coal. However, in the same study, the adsorption of BG on Jamun stem powder was maximum at neutral pH. This may be due to variable nature of different adsorbents and variability in the adsorbate-adsorbent interaction at the surface. The adsorption of other organic compounds was also reported to be higher at low pH (Gupta and Gupta, 2018). Mane et al. (2007) reported that the colour removal of BG using bagasse fly ash was maximum at pH 11. The color reduction was reported to occur due to structural changes in the dye molecule. In the present study, the effect of structural changes has been removed by preparing calibration plots at all the studied pH and the adsorption capacity was observed higher in acidic conditions.
Influence of initial dye concentration
The initial dye concentration (C0) is an important parameter that controls the adsorption process. The influence of initial concentration on BG adsorption using BPAC is presented in Fig. 2d. It is evident from the figure that the adsorbed BG amount per gram of adsorbent is increased with the increase in the initial concentration of dye (Mall et al. 2005). This process also reported two stages of adsorption, i.e. rapid and slow. At a lower concentration of dye, all the active sites at the external surface of BPAC are covered by BG through adsorption by boundary layer and a few sites may remain uncovered. As the concentration increases, the available active sites of the adsorbent (which have a fixed number of active sites) get decreased and the adsorption process becomes slow (Allen et al. 2005).
Influence of temperature
To investigate the influence of temperature for the adsorption of BG on BPAC, the temperature was varied from 30 to 70 °C. The influence of temperature on brilliant green adsorption on BPAC is shown in Fig. 3. The maximum adsorption values were obtained when the temperature was 30 °C using BPAC as adsorbent. The results showed that BPAC could easily remove dye in normal conditions. The results reveal that the percentage removal of BG decreases with respect to rise in temperature, which refers to exothermic nature of dye removal on prepared adsorbent. Rehman et al. (2011) also reported exothermic nature of adsorption of brilliant green dye onto radish peel, jamun stem powder, and coal. Mane et al. (2007) reported endothermic nature of brilliant green adsorption onto bagasse fly ash. The endothermic nature in their study was due to occurrence of intra-particle transport pore diffusion rather than boundary layer adsorption. The adsorption of PAH on activated carbon prepared using sawdust was reported to be endothermic (Kumar and Gupta, 2020). The results do not coincide with the present study, as PAHs are aromatic compounds with no definite charge on their surface, while dyes generally carry a definite charge. The adsorption studies of some textile dyes, such as direct yellow and maxilon blue, on activated carbon developed using coconut shells were reported to be exothermic. With the increase in temperature, a decrease in dye adsorption was attributed to an increase in molecular Brownian movement. Moreover, the intermolecular hydrogen bonds between the adsorbate and adsorbent may also undergo cleavage due to the increase in temperature (Aljeboree et al. 2017; Li et al. 2013).
Adsorption kinetics
Figure 4 shows the plot of log (qe–qt) versus t representing pseudo-first-order model. The plot is a straight line with slope (–k1/2.303) and intercepts log (qe) which allows the calculation for adsorption capacity (qe) and adsorption rate constant (k1) (Nandi et al. 2009). Correlation coefficient for the pseudo-first-order model was found to be 0.9434. The adsorption capacity value evaluated by pseudo-first-order model differs significantly from the experimentally observed model (Table 1). This indicates the less applicability of pseudo-first-order model for BG adsorption onto BPAC.
For pseudo-second-order model, the plot of t/qt versus t is a straight line with 1/qe as slope and intercept as 1/k2qe2 (Fig. 5). The slope and intercept values were used for evaluation of qe and k2, respectively. The correlation coefficient value was tending to unity for the pseudo-second-order model (Table 1). The calculated qe value and the experimental qe value fairly agree with pseudo-second-order model in comparison to the pseudo-first-order model. Therefore, it is evident that BG adsorption on BPAC follows the pseudo-second-order kinetic model. The better applicability of the pseudo-second-order model was also reported in the previous study for adsorption of rhodamine dye onto activated banana peel carbon (Singh et al. 2020). Mane et al. (2007) also found similar observations for the removal of brilliant green dye using bagasse fly ash as adsorbent. Activated carbon prepared from vehicular tyre also shows similar results for adsorption of PAH anthracene from aqueous medium (Gupta, 2018). The pseudo-second-order model was also observed to be applicable during adsorption of malachite green dye on bagasse fly ash as well as activated carbon (Mall et al. 2005).
Adsorption isotherms
Langmuir isotherm explains monolayer adsorption during removal of an adsorbate by any adsorbent. At varying temperatures, the drawn Langmuir isotherm plots were straight lines (Fig. 6). The slopes and intercepts of these plots were used to evaluate Langmuir parameters b and qm. The affinity of binding sites is related to constant ‘b’, which is useful to compare adsorbent affinity with dye molecule. A reduction in values of ‘b’ points out that the decrease in adsorption will occur with increasing temperature (Rehman et al. 2011; Mane et al. 2007; Anwar et al. 2011).
At varying temperatures, the drawn Freundlich isotherm plots were also straight lines (Fig. 7). The slopes and intercepts of these plots were used to evaluate Freundlich parameters n and Kf (Table 2). The values of correlation coefficients indicated that both isotherms are applicable for BG adsorption onto BPAC. Furthermore, Langmuir isotherm was observed to be slightly better compared to Freundlich model. The results suggest that monolayer adsorption of BG on the active sites of BPAC takes place initially, but as soon as the monolayer is completely occupied, the adsorption also occurs in the form of multilayer. The values of Freundlich parameter ‘n’ greater than unity indicate that adsorption of brilliant green onto BPAC is favorable. The values of correlation coefficient were also reported to be comparable for adsorption of BG onto bagasse fly ash (Mane et al. 2007). However, adsorption of rhodamine B dye on powder of banana peel without treatment also follows Langmuir adsorption isotherm (Singh et al. 2018). The adsorption capacities of different adsorbents in varying media are compared in Table 3. It is evident from the comparison data that the adsorption capacity of the adsorbent prepared in present study is higher than most of the tabulated high-quality adsorbents. However, adsorption capacity was slightly lower than the adsorption capacity of graphene, which may be attributed to appearance of nano-scale characteristics of graphene.
Thermodynamic parameters
The value of enthalpy change (∆Ho) for the adsorption of brilliant green dye onto BPAC was found to be negative, indicating the nature of BG adsorption on BPAC as exothermic. The positive entropy change (∆So) value observed during the adsorption process indicates that the adsorption of BG onto BPAC results in an increase in randomness at adsorbent–adsorbate interface and also suggests the adequate affinity of BG towards adsorbent (Gupta and Gupta, 2016; Gupta and Singh, 2018). The negative value of Gibbs energy (∆Go) indicates the spontaneity of the studied adsorption process (Table 4). An increase in negative ∆G0 values indicates that BG adsorption is favorable at lower temperatures. Similar observation was reported by Aljeboree et al. (2017) for adsorption of direct yellow and maxilon blue dyes onto coconut shell based activated carbon.
Globally, several thousand people die every day due to unavailability of pure and safe drinking water. In India, a number of people die each day from diseases and illnesses related to water pollution. The World Health Organization has also assessed the quality of water resources and reported about the heavy contamination in various water bodies. Water pollution is of great concern to environmentalists throughout the world. Water pollution is mainly due to wastewater discharged by dry cleaners and dyeing industries in large quantities. The present study deals with finely ground banana peel powder after activation as activated carbon for fast adsorption and removal of hazardous brilliant green dye from the aqueous solution. Powdered banana peel activated carbon (BPAC) was utilized in the form of bio-sorbent for efficient removal of brilliant green from aqueous phase.
Conclusion
Powdered banana peel activated carbon (BPAC) was utilized in the form of bio-sorbent for removal of brilliant green from aqueous phase. The brilliant green dye was removed quantitatively (> 99%) using 15 mg of the dose of prepared adsorbent. The adsorption was more favorable in the acidic range of pH. The maximum adsorption capacity of BG for BPAC was found to be greater than 900 mg/g at optimum conditions of pH and temperature. The kinetics adsorption was explained through the pseudo-second-order model. Based on temperature effect as well as the enthalpy change value, the adsorption of BG onto BPAC was exothermic. The sorption of BG on BPAC was spontaneous based on the negative values of ∆Go. The positive value of ∆So suggested that the adsorption of BG on activated carbon surface increased randomness. The prepared adsorbent in the present study is highly economical, effective, and environment friendly. The study supports the usefulness of the adsorbent for various industrial treatment systems as well as its commercial production for the removal of various pollutants.
References
Agarwal S, Gupta VK, Ghasemi M, Amin ZA (2017) Peganum harmala-L Seeds adsorbent for the rapid removal of noxious brilliant green dyes from aqueous phase. J Mol Liq 231:296–305
Ali AF, Kovo AS, Adetunji SA (2017) Methylene blue and brilliant green dyes removal from aqueous solution using agricultural wastes activated carbon. J Encapsulation Adsorpt Sci 7:95–107
Aljeboree AM, Alshirifi AN, Alkaim AF (2017) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:S3381–S3393
Allen SJ, Gan Q, Matthews R, Johnson PA (2005) Mass transfer processes in the adsorption of basic dyes by peanut hulls. Ind Eng Chem Res 44:1942–1949
Alver E, Metin AÜ, Brouers F (2020) Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int J Biol Macromol 154:104–113
Anwar J, Shafique U, Zaman W, Nisa Z, Munawar MN, Jamil N, Salman M, Dar A, Rehman R, Saif J, Gul H, Iqbal T (2011) Removal of chromium on Polyalthia longifolia leaves biomass. Int J Phytoremediation 13(5):410–420
Annadurai G, Juang RS, Lee DJ (2002) Factorial design analysis for adsorption of dye on activated carbon beads incorporated with calcium alginate. Adv Environ Res 6(2):191–198
Bahl A, Bahl BS, Advanced organic chemistry, S.S. Chand Publications, 2007
Bhattacharyya KG, Sarma A (2003) Adsorption characteristics of the dye, Brilliant Green on Neem leaf powder. Dyes Pigm 57(3):211–222
Bhattacharya G, Sas S, Wadhwa S, Mathur A, Mclaughlin J, Roy SS (2017) Aloe Vera assisted facile green synthesis of reduced grapheme oxide for electrochemical and dye removal applications. RSC Adv 7:26680–26688
Feng Y, Yang F, Wang Y, Ma L, Wu Y, Kerr PG, Yang L (2011) Basic dye adsorption onto an agro-based waste material – Sesame hull (Sesamum indicum L.). Bioresour Technol 102(22):10280–10285
Fil BA, Karcioglu KZ, Boncukcuoglu R, Yilmaz AE (2013) Removal of cationic dye (Basic Red 18) from aqueous solution using natural Turkish clay. Global NEST J 15(4):529–541
Franco DSP, Fagundes JLS, Georgin J, Salau NPG, Dotto GL (2020) A mass transfer study considering intraparticle diffusion and axial dispersion for fixed-bed adsorption of crystal violet on pecan pericarp (Carya illinoensis). Chem Eng J 397:125423
Ghaedi M, Nasab AG, Khodadoust S, Rajabi M, Azizian S (2014) Application of activated carbon as adsorbents for efficient removal of methylene blue: kinetics and equilibrium study. J Ind Eng Chem 20(4):2317–2324
Ghaedi M, Ansari A, Bahari F, Ghaedi AM, Vafaei A (2015) A hybrid artificial network and particle swarm optimization for prediction of removal of hazardous dye brilliant green from aqueous solution using zinc sulphide. Spectrochim Acta A Mol Biomol Spectrosc 137:1004–1015
Ghasemi M, Ghasemi N, Zahedi G, Alwi SRW, Goodarzi M, Javadian H (2014) Kinetic and equilibrium study of Ni(II) sorption from Aqueous solutions onto Peganum harmala-L. Int J Environ Sci Technol 11(7):1835–1844
Gupta H (2018) Anthracene removal from water onto activated carbon derived from vehicular tyre. Sep Sci Technol 53(4):613–625
Gupta H, Gupta B (2016) Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin Water Treat 57:9498–9509
Gupta H, Gupta B (2018) Vehicular tire as potential adsorbent for the removal of polycyclic aromatic hydrocarbons. Polycycl Aromat Compd 38(4):354–368
Gupta H, Kumar R (2016) Removal of PAH anthracene from aqueous media using banana peel activated carbon. Int J Sci Res Environ Sci 4(4):0109–0114
Gupta H, Kumar R (2020) Distribution of selected polycyclic aromatic hydrocarbons in urban soils of Delhi India. Environ Technol Innov 17:100500
Gupta H, Singh S (2018) Kinetics and thermodynamics of phenanthrene adsorption from water on orange rind activated carbon. Environ Technol Innov 10:208–214
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modelling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 144(2):235–244
Hayeeye F, Sattar M, Chinpa W, Sirichote O (2017) Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf a: Physicochem Eng Asp 513:259–266
Jain SN, Gogate PR (2018) Efficient removal of Acid Green 25 dye from wastewater using activated Prunus dulcis as biosorbent: Batch and column studies. J Environ Manag 210:226–238
Kong L, Qiu F, Zhao Z, Zhang X, Zhang T, Pan J, Yang D (2016) Removal of brilliant green from aqueous solutions based on polyurethane foam adsorbent modified with coal. J Clean Prod 137:51–59
Kumar A, Gupta H (2020) Activated carbon from sawdust for naphthalene removal from contaminated water. Environ Technol Innov 20:101080
Kumar A, Thanki A, Padhiyar H, Singh NK, Pandey S, Yadav M, Yu Z-G (2021a) Greenhouse gases emission control in WWTS via potential operational strategies: a critical review. Chemosphere 273:129694
Kumar A, Jigyasu DK, Kumar A, Subrahmanyam G, Mondal R, Shabnam AA, Cabral-Pinto MMS, Malyan SK, Chaturvedi AK, Gupta DK, Fagodiya RK, Khan SA, Bhatia A (2021b) Nickel in terrestrial biota: comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 275:129996
Kumar A, Pinto MC, Candeias C, Dinis PA (2021c) Baseline maps of potentially toxic elements in the soils of Garhwal Himalayas, India: assessment of their eco-environmental and human health risks. Land Degrad Dev 32(14):3856–3869
Kumar A, Kumar M, Pandey R, ZhiGuo Y, Cabral-Pinto M (2021d) Forest soil nutrient stocks along altitudinal range of Uttarakhand Himalayas: an aid to nature based climate solutions. Environ Technol Innov 22:101405
Kumar A, Sharma MP, Taxak AK (2017) Analysis of water environment changing trend in Bhagirathi tributary of Ganges in India. Desalin Water Treat 63:55–62
Kumar A, Sharma MP, Yadav NS (2015) Assessment of River health of Chambal River based on Biological Communities. India J Mater Environ Sci 6(11):3045–3053
Langmuir I (1918) The Adsorption of Gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li H, Huang G, An C, Hu J, Yang S (2013) Removal of tannin from aqueous solution by adsorption onto treated coal fly ash: kinetic, equilibrium, and thermodynamic studies. Ind Eng Chem Res 52:15923–15931
Liu X, Wang B, Jing G, Sun Y (2019) Adsorption behaviours of methylene blue on sunflower stem pith. Water Sci Technol J Int Assoc Water Pollut Res 79(8):1458–1466
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf A Physicochem Eng Asp 264:17–28
Mane VS, Mall ID, Srivastava VC (2007) Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes Pigm 73:269–278
Moussavi G, Khosravi R (2011) The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste. Chem Eng Res Des 89(10):2182–2189
Namasivayam C, Kavitha D (2002) Removal of congo red from water by adsorption on to activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigm 54(1):47–58
Nandi BK, Goswami A, Purkait MK (2009) Adsorption characteristics of brilliant green dye on kaolin. J Hazard Mater 161:387–395
Pathania D, Sharma S, Singh P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carcia bast. Arab J Chem 10:1445–1451
Rehman R, Abbas R, Ayub A, Ain Q, Salman M, Mahmud T, Shafique U, Zaman W (2011) Comparative study of Brilliant Green dye adsorption from water by radish peels, jamun stem and coal. Elec J Env Agricult Food Chem 1579–4377
Sawant S, Lalge K, Labade S, Khengre N, Burungale A (2017) Effective removal and recovery of fast green FCF dye from wastewater using green adsorbent. Int J Appl Chem 13:341–351
Singh S, Kumar A, Gupta H (2020) Activated banana peel carbon: a potential adsorbent for Rhodamine B decontamination from aqueous system. Appl Water Sci 10(8):1–8
Singh S, Parveen N, Gupta H (2018) Adsorptive decontamination of rhodamine-B from water using banana peel powder: a biosorbent. Environ Technol Innov 12:189–195
Söğüt EG, Karataş Y, Gülcan M, Kılıç NÇ (2020) Enhancement of adsorption capacity of reduced graphene oxide by sulfonic acid functionalization: Malachite green and Zn (II) uptake. Mater Chem Phys 256:123662
Tavlieva MP, Genieva SD, Georgieva VG, Vlaev LT (2013) Kinetic study of brilliant green adsorption from aqueous solution onto white rice husk ash. J Colloid Interface Sci 409:112–122
Vasuki M, Punitha S, Karthika M (2017) A comparative study of adsorption of acid blue 9 and brilliant green from aqueous solution by activated carbon derived from coconut shell and palmyra fruit nut shell. J Adv Appl Sci Res 2454–3225
Zhao D, Qiu Q, Wang Y, Huang M, Wu Y, Liu X, Jiang T (2016) Efficient removal of acid dye from aqueous solutions via adsorption using low-cost blast-furnace slag. Desalin Water Treat 57(58):28486–28495
Acknowledgements
The authors, Santosh Singh and Himanshu Gupta would like to thank the administration of IFTM University Moradabad, U.P., India, for providing necessary facilities to conduct the present work.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that the present study has no conflict of interests and ethical statement to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, S., Gupta, H., Dhiman, S. et al. Decontamination of cationic dye brilliant green from the aqueous media. Appl Water Sci 12, 61 (2022). https://doi.org/10.1007/s13201-022-01596-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01596-5