Abstract
Propolis extracts have been used in traditional medicines since ages due to its advantageous complex chemical composition. However, the antibacterial and antifungal activity of poplar propolis extracts prepared in Natural Deep Eutectic Solvent (NADES) are seldom studied. This study investigates suitable alternate for ethanol as a solvent for extraction for Polish poplar propolis. It also attempts to identify suitable extraction condition for the efficient transfer of compounds from propolis to the solvents. The extraction efficiency of NADES extracts was assessed in terms of total phenolic content, antioxidant activity and antimicrobial activity. The chemical composition of the extracts was analysed using UHPLC-DAD-QqTOF-MS. Four extracts, prepared in Propylene Glycol, Choline Chloride:Propylene Glycol (1:3), Choline Chloride:Propylene Glycol (1:4) and Choline Chloride:Glycerol (1:2), demonstrated activity and properties similar to ethanolic extract and extraction at 50 °C was found the most suitable for propolis. HPLC analysis confirmed that the chemical cocktail extracted by these solvents from propolis were identical with minor variations in their concentration as compared to its ethanolic extract. Thus, extracts of propolis at 50 °C in Propylene Glycol, Choline Chloride:Propylene Glycol (1:3) and Choline Chloride:Propylene Glycol (1:4) can be alternates for ethanolic extracts.
Similar content being viewed by others
Introduction
Propolis is a plant-derived honeybee product used in traditional medicines since ages due to its complex therapeutically relevant chemical composition. Studies have established numerous health benefits like antimicrobial, antioxidant, anti-inflammatory, immunostimulant, hepatoprotective, anti-ulcer, antitumor and wound-healing properties of propolis1,2,3,4. Most of the bioactive molecules identified from it are secondary plant metabolites like phenolics and flavonoids like chrysin, galangin, pinocembrin, pinobanksin etc.5. Thus, the chemical composition of propolis obtained from different geographical area can vary according to the variation in local flora. In this study we focus on propolis collected in Poland (Europe). Typical propolis from temperate zones originates principally from different poplar and aspen buds exudates as well as birch buds. The native species in Poland, are black poplar (Populus nigra L.), European aspen (Populus tremula L.), white poplar (Populus alba L.), silver birch (Betula pendula Roth) and white birch (Betula pubescens Ehrh.)6. The chemical composition of natural products extracts and their derivatives depends on the solvent polarity and method of extraction7,8. Hydroethanolic solutions are the most common solvent for the extraction of propolis. However, there is a need for alternate formulations, as ethanolic extract induces irritability on skin due to its aggressive nature and is also unsuitable for children, pregnant and breastfeeding woman and some religious communities9. Propylene glycol (PG), polyethylene glycol (PEG), glycerol (Gly) and plant oils are a few alternative formulations to alcoholic solutions which are effective, safe and non-toxic in a pharmaceutical perspective10,11,12.
Another alternative extractants are Deep Eutectic Solvents (DES) that have gained popularity in the past decades, not only as a solvent for extraction but also in the fields of bio-catalysis, electrochemistry, CO2 capture and for various biomedical applications13,14,15. DES is a mixture of two or more components which form a single phase with a unique lowered melting point (lower than individual components) and essentially contains a hydrogen bond donors and acceptors. DES composed of primary metabolites are termed Natural DES/NADES16. The use of DES/NADES for natural product extraction aligns with green chemistry principles. The ability to adjust the solvent's polarity through DES composition enhances its extraction capabilities. While NADES has been explored for various natural product extractions, research on its effectiveness for extracting propolis is limited17,18,19,20.
In the current study we attempt to extract different propolis samples in 10 solvent systems in three different extraction conditions; at room temperature for 100 h (the traditional approach used by many beekeepers), at 50 °C for three hours and under ultrasonication for one hour. The solvents include 70% ethanol, neat propylene glycol and different NADES. All the extracts are evaluated for their antioxidant activity, total phenolic content, antibacterial, antifungal activity, and pH. Based on the results of these preliminary studies, five solvents and one method of extraction was finalised. The efficiency of extraction of the selected solvents was confirmed with two new samples of propolis; their antifungal and antibacterial activity were investigated. Further, HPLC analysis of extracts confirmed the extraction of all compounds from propolis in each solvent.
Materials and methods
Chemicals
Choline chloride, L-lysine, propylene glycol, glycerol, DL-lactic acid, citric acid, gallic acid, Na2CO3, 1,1-diphenyl-2- picrylhydrazyl radical (DPPH), Nile red, Folin-Ciocalteu reagent, ferric chloride, 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ), acetic acid, ferrous sulphate, resazurin, were purchased from Merck. Methanol and ethanol were purchased from POCH. Propolis samples were collected from various apiaries located near Gdansk, a city in northern Poland.
Preparation of solvents
10 different solvents were used for the extraction of propolis which included neat propylene glycol, natural deep eutectic solvent (NADES) and 70% (v/v) hydroethanolic solution (as a reference). The composition of the solvent systems is given in Table 1. NADES were prepared by mixing the components in the prescribed ratios with the aid of a magnetic stirrer at 50 °C until a homogenous solution is formed.
Polarity
Polarity of the prepared solvents was determined using the solvatochromic dye Nile red21,22. A 10 mM stock solution of the dye in ethanol was prepared and stored at 4 °C. 20 µL of this stock solution was added to 2 mL of NADES and mixed well in a shaker until a homogeneity is obtained. The absorbance was measured in a cuvette in a UV spectrophotometer in the range 400–700 nm. Pure NADES solvent was used as the blank. The solvent polarity was calculated based on Eq. (1) using the obtained λmax.
where, ENR is the molar transition energy in kcal/mol, h is Plank’s constant, NA is Avogadro number, c is the velocity of light and λmax is wavelength in nm.
Preparation of propolis extracts
Three samples of propolis labelled 1, 2, and 3 were frozen at − 80 °C for 2 h and ground into a fine powder. 0.5 g propolis was then added with 10 mL of solvent and extraction was performed in 3 different conditions (i) at room temperature with shaking (180 rpm) for 100 h (ii) ultrasonicated for 1 h and (iii) at 50 °C with shaking (180 rpm) for 3 h. Upon completion of the prescribed time the content was centrifuged at 10,000 rpm for 20 min to separate the extract from solid residue. The obtained extracts were collected after filtration to remove any solid residues and were stored in dark. The same procedure was adopted for two more samples labelled 4 and 5 which were extracted at 50 °C for 3 h.
Total phenolic content
Total phenolic content of the propolis extract was estimated using the Folin-Ciocalteu method with slight modifications23. 100 μL Folin-Ciocalteu reagent was added with 20 μL propolis extract diluted 100 folds in ethanol. After 5 min of incubation 600 μL Na2CO3 (0.94 M) was added followed by ultrapure water to make up to a final volume of 2 mL. The mixture was incubated for 90 min at ambient temperature and absorbance of the samples were measured at 725 nm. The result was expressed as milligrams of gallic acid equivalence per gram of raw propolis. The experiment was conducted in triplicate.
DPPH assay
The radical scavenging activity of the extracts was measured using DPPH assay following previously reported procedure23. The prepared extracts of propolis were diluted 10 times in methanol prior to the assay. A twofold serial dilution in methanol was adopted to yield a final concentration in a range between 5 and 0.00985% (v/v) and a final volume of 100 µL in a 96-well microtiter plate. 100 µL of 0.2 mM DPPH solution was added to each well with extracts and methanol (control) prepared in triplicate. The microtiter plates were incubated for 30 min in dark at ambient temperature and absorbance was measured at 517 nm. The % of radical scavenging activity was calculated from Eq. (2) from the values obtained for absorbance (ADPPH, AMeOH, ASample). The IC50 value was calculated from % of radical scavenging activity using GraphPad Prism 5.
where, ADPPH, AMeOH and ASample are the values of absorbance measured for of DPPH solution, methanol and sample respectively.
FRAP assay
Antioxidant activity of propolis extract was also assessed using FRAP assay24 with minor changes. In a 96 well microtiter plate 190 µL of FRAP reagent was added with 10 µL propolis extract diluted 50 folds in ethanol and incubated in dark for 30 min. A calibration curve was plotted with 0.5–8 mM solution of Fe2SO4 in acetate buffer. The experiment was conducted in triplicate. The absorbance was measured on a microtiter plate reader at 593 nm.
Determination of minimum inhibitory concentration (MIC)
The antimicrobial activity of all the extracts were tested against gram positive bacteria Staphylococcus aureus ATCC 29213, Staphylococcus aureus ATCC 25923 and four strains of pathogenic fungi of the genus Candida spp., namely: Candida albicans SC5314, Candida glabrata DSM 11226, Candida krusei DSM 6128 and Candida parapsilosis DSM 5784. Serial two-fold broth microdilution method was adopted to investigate the minimum inhibitory concentration. An overnight culture of the cells was used for the preparation of the cell suspension in phosphate buffer solution. The optical density was adjusted to 0.1 at 600 nm for bacterial strains and to 0.1 at 660 nm for the selected fungi variants. The suspensions were diluted 1/100 (v/v) in MHB2 for bacteria and 1/50 (v/v) in RPMI 1640 medium supplemented with MOPS 3.5% (w/v) and 2% (w/v) glucose (pH of the medium was adjusted to 7.0 with solid NaOH) for fungi, to obtain a final cell concentration of approximately 106 CFU/mL (in the case of staphylococci) and 2 × 104 CFU/mL (for yeasts strains). Two-fold serial dilution of propolis extracts in respective mediums in a 96-well microtiter plate followed by the addition of 100 µL of the diluted cell suspensions resulted in final propolis concentration varying between 2.5 and 0.08% (v/v). The plates were incubated at 37 °C for 24 h. The minimum inhibitory concentration (MIC) was estimated by optical density measurements of the plates at 531 nm and confirmed by resazurin test25.
pH measurements
pH of the extracts at a final concentration 2.5% (v/v) of solvent in MHB2 medium and RPMI 1640 supplemented with MOPS and 2% glucose was measured using a pH meter (Mettler Toledo).
Kill-time assay
Time-kill assay was performed with the most active sample of propolis (sample number 4) against C. glabrata DSM 11226 and S. aureus ATCC 25923. An overnight culture of the cells was suspended in sterile water with optical density adjusted to 0.1 at 660 nm and 600 nm for yeasts and staphylococci respectively. Subsequently 1 mL of each suspension was diluted 100 folds in appropriate medium RPMI for C. glabrata and (final cells concentration was approximately 104 CFU/mL) and MHB2 for bacteria (final cells concentration was approximately 106 CFU/mL). The prepared inoculum was divided into five parts of equal volume and was added with 4xMIC of the extracts of the most active sample of propolis (sample 4) and was incubated at 37 °C. 50 μL of each part with appropriate dilutions were spread on a SAB agar plate (C. glabrata) and a Baird-Parker agar plate supplemented with egg yolk tellurite emulsion (S. aureus) at time 0, 2, 5 and 24 h. The plates were incubated overnight and the number of colonies were counted.
UHPLC-DAD-QqTOF-MS analysis
Acetonitrile (both gradient grade and LC/MS grade) and LC/MS grade water as well as formic acid was purchased from Sigma-Aldrich. Analytical standards of caffeic acid, p-coumaric acid, ferulic acid, isoferulic acid, vanillin, pinobanksin, apigenin, kaempferol, chrysin, pinocembrin, sakuranetin, galangin, pinostrobin, pinocembrin chalcone, pinocembrin dihydrochalcone were purchased from Extrasynthese. Ultrapure water (< 0.06 μS/cm) was obtained from Hydrolab HLP20UV (Hydrolab, Straszyn, Poland) purification system. For UHPLC analyses, Thermo Scientific™ UltiMate™ 3000 system (Thermo Scientific™ Dionex™, Sunnyvale, CA, USA) with an autosampler and DAD detector and Compact QqTOF-MS detector (Bruker, Darmstadt, Germany) was applied. Chromatographic separation was performed on Kinetex® C18 polar 2.6 µm, 100 Å, 150 × 2.1 mm analytical column with guard-column (Phenomenex, Torrence, CA, USA) thermostated at 20 ± 1 °C. The injection volume of the sample was set to 1 μL. The mobile phase used for chromatographic separation consisted of 0.1% formic acid solutions in water (solvent A) and acetonitrile (solvent B). The flow rate was set at 0.4 mL/min and the separation was obtained using following gradient: 95% of solvent A isocratic for 10 min, decreasing to 80% within 1 min and held isocratic for another 10 min, decreasing to 65% A within 1 min and held isocratic for 8 min, decreasing to reach 40% A within 15 min and isocratic for another 15 min. Subsequently, the elution solvent increased 100% B, the column was rinsed and then the solvent returned to 95% A. Before the next analysis the system was stabilized. Spectral data was recorded in the 200–600 nm range as well as at 280, 320, 360 nm. MS detector was used in ESI negative mode, ion source temperature was set at 100 °C, nebulizer gas pressure at 2.0 bar, dry gas flow 0.8 L/min and temperature 210 °C. The capillary voltage was set at 2.20 kV and collision energy at 8.0 eV. Internal calibration was obtained by injection of 10 mM solution of sodium formate clusters. For ESI–MS/MS experiments, collision energy was set at 35 eV and nitrogen was used as collision gas. Before the analysis, all the extracts were filtered through CHROMAFIL® 0.2 µm, Ø13mm, H-PTFE syringe filter (Macherey–Nagel, Düren, Germany). Standard solutions were prepared in ethanol and the working standard solutions were diluted in ethanol or in ultrapure water. The calibrations curves were prepared in concentration range concentrations of 6.25–200 µg/mL and the correlation values were 0.9996–1.0000. The content of derivatives of caffeic acid, p-coumaric and pinobanksin were calculated as their equivalents similarly as chalcones, that were calculated as equivalents of pinocembrin chalcone or pinocembrin dihydrochalcone. Additionally, the results were corrected taking into account differences in molecular mass.
Results and discussion
Polarity of solvents
Polarity of the solvent used for extraction qualitatively and quantitatively determines the extraction efficiency. More polar solvents can extract more polar compounds from propolis. The polarity of the NADES prepared are listed in Table 2. The solvatochromic dye failed to dissolve in Lys:H2O. Hence the polarity of only seven NADES could be estimated. The polarity of all the solvents were similar to 70% EtOH. A lower ENR value corresponds to a higher polarity and vice versa, making LA:H2O and CA:PG the most polar solvents. Thus, minor variations in the chemical composition and activity of NADES extracts can be anticipated against their alcoholic reference.
Preliminary analysis
A preliminary study was conducted with three samples of propolis labelled 1, 2, and 3, to determine the solvents suitable for extraction which can exhibit comparable chemical and biological activity to ethanolic extract. It is also an attempt to identify the most suitable mode of extraction for efficient transfer of phenolics from propolis to the solvent regardless of its viscosity. All the solvents used, except Lys:H2O and 70% ethanol, are highly viscous liquids at room temperature. Extractions assisted by ultrasonication or elevated temperatures are advantageous over the conventional method, particularly for viscous deep eutectic solvents. Temperature and viscosity are factors inversely proportional to each other and result in efficient transfer of mass from propolis to the NADES. On the other hand, ultrasound waves produce cavitation bubbles in the solvents leading to increased yield of extraction. However, higher temperature can lead to degradation of polyphenols, and ultrasonication poses the risk of free radical generation26. Thus, using three different methods of extraction can aid in quantitative and qualitative evaluation of the extraction efficacy. It was observed that the extracts of each sample of propolis in different solvents appeared in a spectrum of colours ranging from yellow to brownish black, which can be an indicator of their varying composition and interactions with solvent.
Total phenolic content
Phenolic compounds reduce the phosphomolybdic–phosphotungstic acid present in the Folin-Ciocalteu reagent to form a blue complex whose intensity is dependent on the concentration of phenolics27. The total phenolic content (TPC) of the samples 1, 2 and 3 varied with solvents and extraction conditions. Ethanolic extract of each sample is taken as the reference for the evaluation of TPC for specific extraction conditions. The TPC of the samples 1, 2 and 3 are shown in Fig. 1. It is observed that the amount of phenolics extracted in ethanol is unaffected by the method of extraction (statistically important differences were noted only for propolis 3, but even in the case of this product, the absolute values of phenolics content in extracts obtained using different methods differed slightly—less than 10%). While the method of extraction significantly impacted effectiveness of recovery of phenolics in some other solvents systems, e.g. in the case of propolis sample 1 statistically important differences were observed for all NADES except of CC:PG(1:3). On the other hand, the composition of the solvents also importantly affected the efficiency of phenolics extraction when the same method of extraction process was applied. For about 50% difference in TPC was observed for the extracts of sample 1 obtained with the classical approach (room temperature for 100 h) in 70% (v/v) ethanol and CC:LA:Water (1:1:1). Concluding results of this part of our study, it is revealed that PG and other NADES solvents extracted sufficient amount of phenolics, comparable with the reference ethanol extract. Lys:H2O demonstrated a superior extraction efficiency for sample 3 over others (independent of the method of extraction). However, no method of extraction guaranteed consistent high yield of phenolics in all three tested samples of propolis.
Antioxidant activity
Oxidation processes have different mechanisms, demanding more than one method for a better understanding, herein we assess the antioxidant activity by FRAP assay and DPPH assay. The FRAP assay is a widely used method to understand the antioxidant potential. However, all the compounds responsible for the conversion of Fe3+ to Fe2+ are not necessarily antioxidants28. Thus, FRAP assay along with DPPH assay were used and the results are summarised in Figs. 2 and S1. DPPH assay gives a quantitative measure of the total antioxidant potential. However, analysis of the results of this assay (Fig. S1) led to general conclusion that this method is not suitable for the determination of the antioxidant potential of propolis extracts produced with NADES. Mainly because of observed huge differences in the values of antioxidant potential of extracts produced with different solvents, and also (which is even more important) extracts obtained in the same solvent but using different methods of extraction. In some cases these differences were at the level 400 or even 600%, e.g. extracts of propolis 3 produced in CA:PG (1:4) or LA:Water (85:15). These differences completely do not correspond to the results of the assays aiming in determination of the total phenolics content and also FRAP method. The appropriate control measurements revealed that the solvents used for extraction alone do not affect the results of the assay and no important differences in DPPH reducing potential of ethanol extracts produced with different methods were observed. This all together can suggest that other than phenolics, ingredients affecting DPPH, were extracted from raw material samples with different solvents and/or under different conditions of the process of extraction. A bit better results were obtained for FRAP method, particularly for samples 1 and 2 (Fig. 2a,b) which showed results comparable to its alcoholic counterpart. Figure 2c indicates that most extracts of sample 3 generally showed a superior antioxidant activity than their corresponding ethanolic extract and some important differences are noted for extracts obtained in the same solvent but with different methods. This observation also suggests that some other compounds/antioxidants, not only phenolics, are recovered from the raw materials and propolis number 3 contains the largest amount of these ingredients. However, it is well proven that the health-promoting potential of propolis results mainly from the content of phenolic compounds, so in our opinion determination of antioxidant potential (either with FRAP or DPPH method) should not be recommended for indirect assessment of the health-beneficial potential of NADES extracts of propolis.
Antimicrobial activity
Determination of minimum inhibitory concentration against C. albicans
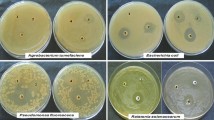
MIC values against C. albicans are listed in Table 3. The extracts produced from propolis sample assigned with number 1 exhibited the highest antifungal potential. Interestingly, the PG extract showed similar antifungal (in two cases slightly higher) effectiveness in inhibition of C. albicans growth as the reference extracts produced with 70% (v/v) ethanol. However, from the point of view of the main goal of this study—assessment of possibilities of using NADES for preparing propolis extracts, the most important observation is that the extracts prepared with five out of eight NADES, namely LA:H2O, CA:PG, CC:PG (1:3), CC:PG (1:4) and CC:LA:H2O (1:1:1) also showed satisfactory, usually only twice lower, antifungal activity compared to reference hydroalhoholic extract. In our opinion these formulations of propolis can be also considered for the treatment of skin and mucous membranes candidiasis. Despite their lower activity, they are free from other disadvantages of alcohol extracts of propolis, of which the irritating properties and burning taste are the most important. The extracts prepared with three other solvents, particularly Lys:H2O exhibited lower antifungal potential. MIC assay when performed with pure solvents indicated no inhibition in the growth of C. albicans leading to the conclusion that the antifungal activity of the extracts in all the solvents are exclusively from the compounds extracted from propolis.
Determination of minimum inhibitory concentration against S. aureus
Propolis extracts are known for its activity against gram positive bacteria. The MIC of all the extracts against S. aureus ATCC reference strain is listed in Table 4. The results are expressed as v/v %, which predominantly varied between 0.625 and 0.156%. All the extracts showed MIC values comparable with alcoholic extract except Lys:water extract. CC:Gly was the least active amongst the solvents which can be attributed to its high viscosity. However, in contrary to C. albicans it was noticed that some of the prepared solvents (containing lactic or citric acid) also exhibited antistaphylococcal activity, with MIC values from 0.312 to 0.625% (v/v). This result can be explained by the fact that these solvents importantly affect acidity of the MHB medium (Table 4). Previous studies have proved that S. aureus is sensitive to pH29. Thus, the inhibition caused by extracts prepared in lactic acid and citric acid can partly be an attribute of the pH alteration they cause.
pH and stability
pH of the bacterial and fungal culture medium after addition of the solvent (final concentration 2.5% v/v) is listed in Table 5. All the solvents containing lactic acid and citric acids showed significantly low pH, making them extremely acidic for practical application. An attempt to increase their pH requires dilutions to an extent that will surpass the MIC of extracts produced in these solvents. Thus, solvents which do not interrupt the pH are selected for further studies. Another key observation was regarding the stability of the extracts. The extracts prepared in LA:H2O (all extraction condition) and ultrasonicated extracts in CC:LA:H2O (1:1:1), CC:LA:H2O (1:2:2), CC:PG (1:3) and CC:PG (1:4) of all propolis samples developed turbidity or precipitates within an observation period of two months. On the other hand, extracts prepared in room temperature and at 50 °C were more resistant to precipitation. The phenolic content in the extracts prepared in 70% EtOH, PG, CC:PG(1:3), CC:PG(1:4) and CC:Gly(1:2) in room temperature and elevated temperature is comparable. However, extraction at 50 °C can be considered superior to the other when the time of extraction is accounted. Further, the effect of temperature can be utilised to reduce the viscosity of solvents for better extraction.
Evaluation of samples 4 and 5
Based on the preliminary screening of extraction method and solvent to be used, extractions at 50 °C for 3 h in five solvents systems—(i)70% EtOH, (ii) PG, (iii) CC:PG (1:3), (iv) CC:PG (1:4) and (v) CC:Gly (1:2) was finalised to be used for the extraction of two more samples of propolis labelled 4 and 5. This part of research was performed to finally confirm possibility of preparing alternative formulations of propolis with selected NADES that exhibit antimicrobial potential (both antibacterial and antifungal) comparable to hydroalcoholic extracts. Moreover, we decided to determine antimicrobial activity of the extracts against other pathogenic species of Candida spp., namely: C. glabrata, C. krusei and C. parapsilosis. Antibacterial activity was also investigated with one additional reference S. aureus strain. Unfortunately, we were very limited with the amount of the propolis samples 1–3. Thus, we decided to perform this part of the project with two additional samples (assigned as 4 and 5) of propolis. The total phenolic content, antioxidant potential and antimicrobial activity of these extracts were compared with its alcoholic extract and HPLC analysis was performed to identify its chemical composition.
Total phenolic content and antioxidant activity
It is reported that NADES essentially extracts all the compounds present in 70% ethanolic extract in different proportion if they are of similar polarity30. The total phenolic content of sample 4 and 5 are tabulated in Table 6 and varied between 265.30 ± 9.94 mg GAE/g to 210.46 ± 21.9 mg GAE/g and 314.17 ± 9.12 mg GAE/g to 210.46 ± 6.36 mg GAE/g respectively. PG and CC:PG(1:3) showed better efficiency of extraction of phenolics in sample 5 than its alcoholic counterpart while they showed the least efficiency in sample 4. CC:Gly which generally exhibited lower phenolic content maintained the trend in sample 5 while it proved to be better than its PG and CC:PG counterparts in sample 4. CC:Gly is the most viscous solvent among the used solvents, justifying its lower extraction efficacy. A similar effect of viscosity was demonstrated by CC:PG (1:3) and CC:Gly against Sideritis scardica, and Plantago major, two medicinal plants22. A lower extraction efficiency of CC:Gly was also observed for propolis obtained from Bulgaria extracted by ultrasonication30. Another study conducted by Tzani et al. quantified a lower amount of phenolics from Greek propolis extracted in different NADES by ultrasonication31. Further, a study conducted by Alpat and co-workers identified NADES, which extracted upto 65% of phenolics from propolis when compared to its 80% ethanolic extract20.
Because of the previously mentioned disadvantages of the DPPH assay the antioxidant potential of the extracts of propolis samples 4 and 5 was carried out only using the FRAP method and phenolics concentration was determined with the Folin-Ciocalteu reagent. The results are listed in Table 6. The first important observation from this part of the study is the fact that the FRAP assay demonstrated superior antioxidant activity of ethanolic extract, whilst PG and CC:PG (1:3) extracts of sample 5 contained slightly higher concentrations of phenolics compared to hydroalcoholic extract. Considering the extracts prepared in particular solvents the products containing larger concentrations of phenolics exhibit higher antioxidant potential. However, in the case of CC:PG (1:3) the proportions are slightly different than in the case of other solvents.
Antimicrobial activity
Previous studies have shown that propolis inhibits the filamentation of C. albicans32,33 which can reduce the virulence potential and reduce the formation of biofilms. Polyphenols in propolis can interrupt the cell wall formation of fungi by forming complexes with proteins in the cell wall involved in chitin synthesis34. This theory was later confirmed35. Antifungal activity of propolis examined in this study is assessed by estimating the MIC value of each extract against C. albicans, C. glabrata, C. krusei and C. parapsilosis reference strains and the results are tabulated in Table 7. Both the sets of extracts were more effective against C. glabrata, C. krusei and C. parapsilosis as compared to C. albicans. Despite the higher phenolic content of extracts of sample 5, it showed lower antifungal activity when compared to sample 4. The results indicate that all these extracts can act as an alternate for ethanolic extract against the tested species as they exhibit comparable (maximum two times higher value of MIC) activity. However, the extracts produced with CC:Gly exhibit a bit lower antifungal potential.
Several mechanisms of antibacterial activity of propolis are identified like inhibition of (i) synthesis of nucleic acid, (ii) energy metabolism, (iii) cell membrane proteins; alteration of (i) function of cytoplasmic membrane, (ii) ability to form biofilms, (iii) membrane permeability; and depressing bacterial resistance36. But the mechanistic action depends on both the antibacterial component and bacterial strain. The MIC values denoting the antibacterial activity tested against S. aureus ATCC 29213 and S.aureus ATCC 25923 is given in Table 7. All extracts of 4 and 5 showed the same MIC value excluding CC:Gly which required twice the concentration of other extracts to attain MIC against S. aureus ATCC 29213. The same trend was observed for extracts of sample 5 against S. aureus ATCC 25923 and agrees with the results obtained for Candida spp. (presented above) and also presented by the study conducted by Trusheva et al. PG showed the least MIC against S. aureus ATCC 25923. Very few studies have focussed on the antibacterial activity of NADES extracts of propolis. Hence it can be concluded the used solvents can be a suitable alternative for ethanolic extract against the given bacterial strains.
Kill time assay
Kill time assay of all 5 extracts of sample 4 was performed at a concentration of 4xMIC against C. glabrata and S. aureus ATCC 25923 (Fig. 3). The results indicate the fungicidal activity of ethanolic extract and CC:Gly extract and growth inhibition by PG and both CC:PG extracts. An increase in cell viability of C.glabrata was observed in the PG and CC:PG extracts after 5 h, until when the growth rate reduced. The extract produced with CC: Gly combination as a solvent exhibited the highest MIC value. Thus, in the case of the kill time assay, this product was used at two times higher concentration compared to other extracts (in each case it was 4xMIC), which partly can explain observed differences in antifungal activity against Candida spp. strains tested. Results indicate that the antimicrobial activity of propolis extract against S.aureus ATCC 25923 is primarily growth inhibition at a concentration of 4xMIC. This observation is well in agreement with previous studies wherein ethanolic extracts of propolis were tested against the same strain23. CC:PG (1:4) extracts showed better inhibition than ethanolic extract followed by CC:Gly and PG extract. However, the differences were not large.
HPLC analysis of the extracts of propolis samples 4 and 5
A total of 32 major compounds were identified in the propolis extracts by UHPLC-DAD-QqTOF-MS and quantified by UHPLC-DAD (Table 8, Fig. S2—Supplementary materials). The comparison of chromatographic profiles of different extracts is presented in Fig. S2. The chemical profiles of the samples were characterized by a variety of flavonoids (flavanones: pinocembrin, pinostrobin, sakuranetin; flavanonols: pinobanksin; flavones: apigenin, chrysin; flavonols: galangin, kampferol). Other relevant compounds included phenylpropanoid derivatives, mostly p-coumaric, caffeic, ferulic, isoferulic acids and their esters. The compounds were identified based on exact mass, MS2 fragmentation and UV spectra as well as reference compounds. Determination of phenylpropanoid derivatives was confirmed by the presence of appropriate losses and diagnostic ions (p-coumaroyl: 163/145, caffeoyl: 179/161, feruloyl: 193/175) in MS2 spectra of their esters. Based on the occurrence of specific components it is possible to confirm the probable botanical origin of the analyzed propolis samples. Both samples contained relevant amounts of phenylopropanoid glycerides (1,3-di-p-coumaroylglycerol, 2-acetyl-1,3-di-p-coumaroylglycerol, 2-acetyl-3-p-coumaroyl-1-feruloylglycerol) characteristic for aspen bud exudates6,37. On the other hand, the investigated samples contained also methylbutenyl esters of caffeic acids and caffeic acid phenethyl ester (CAPE) along with pinocembrin, chrysin, pinobanksin and pinobanksin acetate, characteristic i.a. for black poplar (P. nigra) and not present in aspen6,37. Occurrence of sakuranetin in the investigated samples may be related to black poplar, aspen or white birch, however, its high abundance suggests, it is related rather to white birch (B. pendula). It may be confirmed also by the presence of some amount of compounds characterized by pseudomolecular ions [M−H]− peaks at m/z 365.2114 fragmenting (MS2) to 162(163)/145/118 (p-coumarate) which may be tentatively attributed to hydroxyl-β-caryophyllene p-coumarates characteristic for white birch buds6,37.
The total amount of the quantified major compounds in different propolis extracts ranged from 125.8 to 209.5 mg/g propolis (sample 4) and 222.6 to 406.8 mg/g propolis (sample 5). In both cases, the EtOH extract contained the highest amounts of compounds while CC:Gly extract contained the lowest amounts (nearly two times less). This result explains higher MIC values of the extracts produced with CC:Gly (Table 7) used as a solvent. As mentioned above surprisingly high antimicrobial efficiency of this extract in the kill-time assay performed for C. glabrata is probably a consequence of using higher final concentration of this product(Fig. 3). Considering individual ingredients CC:Gly extract contained importantly lower concentration of p-coumaric acid derivatives (among phenolic acid and benzene derivatives) and some pinobanksin derivatives (among flavonoids and chalcones) compared to extract produced with other solvents, particularly EtOH. Other extracts (PG, CC:PG (1:3), CC:PG (1:4)) were comparable with EtOH and contained 74.1–89.9% of the content in EtOH extracts. This result finally confirms that these three NADES and also PG can be considered as alternatives for ethanol as a solvent for extraction for polish poplar propolis.
However, there were more notable differences in the extraction of individual compounds in favour of EtOH, particularly in the case of chrysin, galangin and caffeic acid. On the other hand, vanillin was extracted better by PG, CC:PG (1:3) and CC:PG (1:4) than by EtOH.
Conclusion
The outcomes of our study revealed that NADES can be considered as an ethanol alternative for preparing propolis extracts. Among eight studied NADES, compositions of choline chloride and propylene glycol (CC:PG(1:3) and CC:PG(1:4)) have been found to be as most suitable alternative extraction solvents. The research in this area should be continued. Many other options for preparing NADES are available and we believe that some of them will enable the preparation of extracts with high health-promoting potential (including antimicrobial activity) and safe for patients.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Banskota, A. H., Tezuka, Y. & Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 15, 561–571 (2001).
McLennan, S. V. et al. The anti-inflammatory agent Propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen. 16, 706–713 (2008).
Sforcin, J. M. & Bankova, V. Propolis: Is there a potential for the development of new drugs?. J. Ethnopharmacol. 133, 253–260 (2011).
Zullkiflee, N., Taha, H. & Usman, A. Propolis: Its role and efficacy in human health and diseases. Molecules 27, 6120 (2022).
Huang, S., Zhang, C.-P., Wang, K., Li, G. & Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 19, 19610–19632 (2014).
Isidorov, V. A., Szczepaniak, L. & Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 142, 101–106 (2014).
Nawaz, H., Shad, M. A., Rehman, N., Andaleeb, H. & Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 56, e17129 (2020).
Rivera-Yañez, N. et al. The role of propolis as a natural product with potential gastric cancer treatment properties: A systematic review. Foods 12, 415 (2023).
Michalak, L. & Trocki, K. Alcohol and Islam: An overview. Contemp. Drug Probl. 33, 523–562 (2006).
Galeotti, F., Maccari, F., Fachini, A. & Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 7, 41 (2018).
Ramanauskienė, K., Inkėnienė, A. M., Petrikaitė, V. & Briedis, V. Total Phenolic content and antimicrobial activity of different Lithuanian propolis solutions. Evid. Based Complement. Altern. Med. 2013, 1–5 (2013).
Kubiliene, L. et al. Comparison of aqueous, polyethylene glycol-aqueous and ethanolic propolis extracts: Antioxidant and mitochondria modulating properties. BMC Complement. Altern. Med. 18, 165 (2018).
Carriazo, D., Serrano, M. C., Gutiérrez, M. C., Ferrer, M. L. & Del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 41, 4996 (2012).
P.M., A., Rodriguez, O. & A., E. New Generations of Ionic Liquids Applied to Enzymatic Biocatalysis. In Ionic Liquids—New Aspects for the Future (ed. Kadokawa, J.) (InTech, 2013). https://doi.org/10.5772/51897.
Zaijun, L., Xiulan, S. & Junkang, L. Ionic liquid as novel solvent for extraction and separation in analytical chemistry. In Ionic Liquids: Applications and Perspectives (ed. Kokorin, A.) (InTech, 2011). https://doi.org/10.5772/14250.
Paiva, A. et al. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2, 1063–1071 (2014).
Dos Santos, C. M., De Souza Mesquita, L. M., Braga, A. R. C. & De Rosso, V. V. Red propolis as a source of antimicrobial phytochemicals: Extraction using high-performance alternative solvents. Front. Microbiol. 12, 659911 (2021).
Funari, C. S. et al. Natural deep eutectic solvents and aqueous solutions as an alternative extraction media for propolis. Food Res. Int. 125, 108559 (2019).
Vasileva, B. et al. Natural deep eutectic extracts of propolis, Sideritis scardica, and Plantago major reveal potential antiageing activity during yeast chronological lifespan. Oxid. Med. Cell. Longev. 2022, 1–19 (2022).
Alpat, U., Nar, T., Karasu, S. & Sagdic, O. Optimization of propolis extraction with natural deep eutectic solvents using central composite design. Phytochem. Lett. 58, 49–59 (2023).
Craveiro, R. et al. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 215, 534–540 (2016).
Grozdanova, T. et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 14, 73 (2020).
Grecka, K. et al. The anti-staphylococcal potential of ethanolic polish propolis extracts. Molecules 24, 1732 (2019).
Bolanos De La Torre, A. A. S., Henderson, T., Nigam, P. S. & Owusu-Apenten, R. K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food. Chem. 174, 119–123 (2015).
Grecka, K. et al. Effect of ethanol extracts of propolis (EEPs) against Staphylococcal Biofilm—Microscopic Studies. Pathogens 9, 646 (2020).
Ruesgas-Ramón, M., Figueroa-Espinoza, M. C. & Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 65, 3591–3601 (2017).
Malta, L. G. & Liu, R. H. Analyses of total phenolics, total flavonoids, and total antioxidant activities in foods and dietary supplements. In Encyclopedia of Agriculture and Food Systems 305–314 (Elsevier, 2014). https://doi.org/10.1016/B978-0-444-52512-3.00058-9.
Prior, R. L. & Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods1. Free Radic. Biol. Med. 27, 1173–1181 (1999).
Lu, L.-C., Chen, Y.-W. & Chou, C.-C. Antibacterial activity of propolis against Staphylococcus aureus. Int. J. Food Microbiol. 102, 213–220 (2005).
Trusheva, B., et al. “Green” approach to propolis extraction: Natural deep eutectic solvents. http://www.proceedings.bas.bg/DOI/2019_7_06.html (2019) https://doi.org/10.7546/CRABS.2019.07.06.
Tzani, A. et al. Green extraction of greek propolis using natural deep eutectic solvents (NADES) and incorporation of the NADES-extracts in cosmetic formulation. Sustain. Chem. 4, 8–25 (2022).
Andrade, J. K. S., Denadai, M., De Oliveira, C. S., Nunes, M. L. & Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 101, 129–138 (2017).
De Castro, P. A. et al. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet. Biol. 60, 74–86 (2013).
Bowman, S. M. & Free, S. J. The structure and synthesis of the fungal cell wall. BioEssays 28, 799–808 (2006).
Corrêa, J. L. et al. Propolis extract has bioactivity on the wall and cell membrane of Candida albicans. J. Ethnopharmacol. 256, 112791 (2020).
Almuhayawi, M. S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 27, 3079–3086 (2020).
Okińczyc, P. et al. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 23, 1262 (2018).
Acknowledgements
The studies were financed by the grant UMO-2020/39/B/NZ7/02901 from the National Science Centre, Poland.
Author information
Authors and Affiliations
Contributions
Jeslin Cheruvathoor Jenny performed experiments and analysed the obtained data. Piotr Marek Kuś performed the HPLC analyses. Piotr Szweda conceptualized the study, reviewed the research proposal and supervised the study. All the authors participated in writing and giving feedback on the manuscript, and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jenny, J.C., Kuś, P.M. & Szweda, P. Investigation of antifungal and antibacterial potential of green extracts of propolis. Sci Rep 14, 13613 (2024). https://doi.org/10.1038/s41598-024-64111-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64111-7
- Springer Nature Limited