Abstract
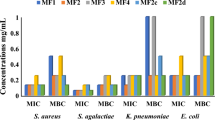
A study was conducted to examine the phytochemical and antibacterial potential of non-polar solvent (hexane, petroleum ether, chloroform, and toluene) based Camelina sativa cv. Calena (EC643910) seed extracts for future investigation into the field of pharmacology, phytochemistry, ethnobotany and other biological actions for drug discovery. Among all tested extracts, hexane (disc 1) showed no inhibition zone against any pathogens, while petroleum ether based camelina seed extract (disc 2) showed sensitive activity with inhibition zones ranging from ~12 to 20 mm against most of the tested pathogens. Similarly, chloroform extract (disc 4) also showed significant activity with inhibition zones ranging from ~11 to 21 mm against all tested pathogens. Toluene extract showed no inhibition zone against Bacillus pumilus, Bacillus subtilis, Bacillus thurengenensis, Pseudomonas fluorescens, but showed significant inhibition zones ranging from ~13 to 18 mm, against Escherichia coli, Agrobacterium tumefaciens and Ralstonia solanacearum. The inhibition zones ranged from 9 to 29 mm for antibacterial activity against standard drug (disc 3 and 6). Chloroform extract showed highest phenolic (~3–4 µg g−1 dry wt), terpene (~2.6 µg of linalool g−1 dry wt), alkaloid (5 µg of atropine g−1 dry wt) and free amino acid (6.4 µg g−1 dry wt) accumulation, whereas petroleum ether extract showed highest (21.5 %) free radical scavenging property. Based on the phytochemical and antibacterial potential with different extracts, camelina was identified as the most suitable source for drug discovery.


Similar content being viewed by others
References
Abramovic, H., Butinar, B., & Nikolic, V. (2007). Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chemistry, 104, 903–909.
Ainsworth, E. A., & Gillespie, K. M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature Protocols, 2, 875–877.
Alabouvette, C., Olivain, C., & Steinberg, C. (2006). Biological control of plant diseases: the European situation. European Journal of Plant Pathology, 114, 329–341.
Bauer, A. W., Kirby, W. M., Sherris, J. C., & Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 45, 493–496.
Bebber, D. P., Ramotowski, M. A. T., & Gurr, S. J. (2013). Crop pests and pathogens move polewards in a warming world. Nature Climate Change, 3, 985–988.
Berhowa, M. A., Polat, U., Glinski, J. A., Glensk, M., Vaughn, S. F., Isbell, T., et al. (2013). Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Industrial Crops and Products, 43, 119–125.
Cal, A. D., Larena, I., Guijarro, B., & Melgarejo, P. (2012). Use of biofungicides for controlling plant diseases to improve food availability. Agriculture, 2, 109–124.
Conn, K. L., Browne, L. M., Tewari, J. P., & Ayer, W. A. (1994). Resistance to Rhizoctonia solani and presence of antimicrobial compounds in Camelina sativa roots. Journal of Plant Biochemistry and Biotechnology, 3, 125–130.
Czochra, M. P., & Widensk, A. J. (2002). Spectrophotometric determination of H2O2 activity. Analytica Chimica Acta, 452, 177–184.
Eidhin, D. N., Burke, J., & O’Beirne, D. (2003). Oxidative stability of omega3-rich camelina oil and Camelina oil-based spread compared with plant band fish oils and sunflower spread. Journal Food Science, 68, 345–353.
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., et al. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature, 484, 186–194.
Francis, A., & Warwick, S. I. (2009). The biology of Canadian weeds. Camelina alyssum (Mill.) Thell.; C. microcarpa Andrz. ex DC.; C. sativa (L.) Crantz. Canadian Journal of Plant Science, 89, 791–810.
Ghorai, N., Chakraborty, S., Gucchait, S., Saha S. K., & Biswas, S. (2012). Estimation of total terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. http://www.nature.com/protocolexchange/protocols/2529#/authorinformation
Ghosh, A. (2006). Antifungal properties of haem peroxidase from Acorus calamus. Annals of Botany, 98, 1145–1153.
Gutierrez-Lugo, M. T., Singh, M. P., Maiese, W. M., & Timmermann, B. N. (2002). New antimicrobial cycloartane triterpenes from Acalypha communis. Journal of Natural Products, 65, 872–875.
Ho, K. Y., Tsai, C. C., Huang, J. S., Chen, C. P., Lin, T. C., & Lin, C. C. (2001). Antimicrobial activity of tannin components from Vaccinium vitisidaea L. Journal of Pharmacy and Pharmacology, 53, 187–191.
Hou, A. J., Liu, Y. Z., Yang, H., Lin, Z. W., & Sun, H. D. (2000). Hydrolyzable tannins and related polyphenols from Eucalyptus globulus. Journal of Asian Natural Products Research, 2, 205–212.
Katerere, D. R., Gray, A. I., Nash, R. J., & Waigh, R. D. (2003). Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry, 63, 81–88.
Kawano, T. (2003). Roles of the reactive oxygen species-generating peroxidase reactions in plant defence and growth induction. Plant Cell Reports, 21, 829–837.
Kumar, K., Gupta, S. M., Arya, M. C., & Nasim, M. (2015a). In vitro antimicrobial and antioxidant activity of Camelina seed extracts: As potential source of bioactive compounds. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. doi:10.1007/s40011-015-0631-9
Kumar, K., Gupta, S. M., Arya, M. C., & Nasim, M. (2015b). Osmolyte and antioxidant adjustments in indoor plants in response to varying low temperature stress. Indian Journal of Plant Physiology, 20, 380–384.
Lee, W. H., & Safinar, I. I. (2012). Antioxidant activity of total phenolics and total flavonoids of Syzygium polyanthum (Wight) Walp leaves. International Journal of Medicinal and Aromatic Plants, 2, 219–228.
Manohar, V., Ingram, C., & Gray, J. (2001). Antifungal activities of origanum oil against Candida albicans. Molecular and Cellular Biochemistry, 228, 111–117.
Moore, S., & Stein, W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. The Journal of Biological Chemistry, 176, 367–388.
Nayanakantha, N. M. C., Rawat, S., Ali, S., & Grover, A. (2014). Defense gene induction in Camelina sativa upon Alternaria brassicae challenge. Indian Phytopathology, 67, 252–256.
Oerke, E. C. (2006). Crop losses to pests. Journal of Agricultural Science, 144, 31–43.
Pearce, G., Strydom, D., Johnson, S., & Ryan, C. A. (1991). A polypeptide from tomato leaves induces wound inducible protienase inhibitor proteins. Science, 253, 895–898.
Pedras, M. S., Yaya, E. E., & Glawischnig, E. (2011). The phytoalexins from cultivated and wild crucifers: Chemistry and biology. Natural Product Reports, 28, 1381–1405.
Puupponen-Pimia, R., Nohynek, L., Meier, C., Kahkonen, M., Heinonen, M., Hopia, A., et al. (2001). Antimicrobial properties of phenolic compounds from berries. Journal of Applied Microbiol, 9, 494–507.
Randhir, R., Tong, L., & Shetty, K. (2004). Stimulation of phenolics, antioxidant and antimicrobial activity in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochemistry, 39, 637–646.
Schanderi, S. H. (1970). Methods in food analysis. New York: Academic Press.
Shamsa, F., Monsef, H., Ghamooshi, R., & Verdian-rizi, M. (2008). Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai Journal of Pharmaceutical Sciences, 32, 17–20.
Soxhlet, F. (1879). Die genichtsanalytische bestimmung des miichfettes. Dingler’s Polytechnisches Journal, 232, 461.
Srinivas, K. V., Rao, K. Y., Mahender, I., Das, B., Krishna, R. K. V., Kishore, H. K., et al. (2003). Flavanoids from Caesalpinia pulcherrima. Phytochemistry, 63, 789–793.
Vukovic, N., Milosevic, T., Sukdolak, S., & Solujic, S. (2007). Antimicrobial activities of essential oil and methanol extract of Teucrium montanum. Evidence-Based Complementary and Alternative Medicine, 4, 17–20.
Wanjala, C. C., Juma, B. F., Bojase, G., Gashe, B. A., & Majinda, R. R. (2002). Erythrinaline alkaloids and antimicrobial flavonoids from Erythrina latissima. Planta Medica, 68, 640–642.
Wink, M. (1988). Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theoretical and Applied Genetics, 75, 225–233.
Wittstock, U., & Gershenzon, J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology,. doi:10.1016/S1369-5266(02)00264-9.
Acknowledgments
The financial support from Defence Research and Development Organization (DRDO) is gratefully acknowledged. The authors wish to thank Director DIBER–DRDO for providing logistics and research facility for successful conduction of the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, K., Pathak, R. Phytochemical analysis and assessment of in vitro antibacterial activity of non-polar solvent based Camelina seed extracts. Ind J Plant Physiol. 21, 255–262 (2016). https://doi.org/10.1007/s40502-016-0223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-016-0223-6