Abstract
The numbers of infections caused by Gram-negative bacteria (GNB) that produce extended-spectrum beta-lactamases (ESBLs) and those that are carbapenem resistant, especially Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae), are increasing, and these infections are becoming a global public health problem. The aim of this study was to assess the prevalence of infections caused by ESBL-producing and carbapenem-resistant Gram-negative bacilli in patients hospitalized at An-Najah National University Hospital in Nablus, Palestine, and to provide healthcare workers with valuable information on the treatment of these infections. A retrospective cross-sectional investigation was conducted at a large tertiary care teaching hospital. The study included patients admitted to the hospital between January and December 2021, from whom ESBL-producing and carbapenem-resistant Gram-negative bacilli were isolated. The patients' clinical and demographic information was obtained from the hospital information system. In addition, information regarding the bacterial isolates and antibiotic resistance was obtained from the hospital's microbiology laboratory. This study included a total of 188 patients—91 males (48.4%) and 97 females (51.6%). The general surgical ward accounted for the highest proportion of infections (30.9%), followed by the surgical ICU (12.2%). The most common infections were caused by ESBL-producing E. coli, which accounted for 62.8% of the cases. Among them, urinary tract infections caused by this microorganism were the most prevalent (44.7% of patients). Over 50% of the patients (54.2%) had a history of antibiotic use, and 77.8% had been hospitalized within the past three months. ESBL-producing E. coli was significantly isolated from blood cultures (p-value = 0.000), and CR-K. pneumoniae was significantly isolated from endotracheal isolates (p-value = 0.001). This study emphasizes the concerning frequency of healthcare-acquired infections caused by ESBL-producing and carbapenem-resistant GNB in a tertiary care hospital. The substantial prevalence of antibiotic resistance presents considerable obstacles to the successful administration of routinely employed antibiotics. The results highlight the immediate need for improved antimicrobial stewardship and the implementation of infection control strategies to reduce the effects of multidrug-resistant GNB on patient well-being and public health.
Similar content being viewed by others
Introduction
The global issue of pathogenic organisms developing antibiotic resistance has grave implications for treating infectious diseases1,2. This phenomenon is primarily caused by the excessive or improper use of antibiotics in human medicine, agriculture, and veterinary medicine3,4. There is an alarming increase in antibiotic resistance among community- and hospital-acquired pathogens5,6.
Continuous exposure of bacterial isolates to a variety of β-lactam antibiotics has resulted in the constant production and mutation of β-lactamases, rendering these bacteria resistant to newly developed β-lactam antibiotics; thus, they are called extended-spectrum β-lactamase (ESBL)-producing pathogens7. Treatment of infections induced by these multidrug-resistant organisms is a major concern among scientists8. There has been a global increase in ESBL-related infections in recent years9. Globally, Gram-negative bacteria are predominantly resistant to β-lactam antibiotics, which are the most common therapeutic option for treating bacterial infections. ESBL-producing Gram-negative bacilli (GNB) can colonize patients without causing acute infection, suggesting that the bacteria are present in certain body sites, such as the nasal and anal cavities, without causing any symptoms10. When the immune system is compromised, these organisms become active and cause infection, accompanied by symptoms such as fever, congestion and painful urination. Escherichia coli and Klebsiella pneumoniae are the most common ESBL-producing pathogens11.
E. coli and K. pneumoniae are among the most common pathogens that cause urinary tract infections. However, K. pneumoniae is more strongly associated with catheter-associated urinary tract infections, while E. coli is more strongly associated with urogenital problems such as urinary tract stones, neurogenic bladder, or obstructive uropathy12.
In 1983, strains that produce ESBL were first reported in Germany. Nonetheless, developing countries have a higher prevalence and thus greater impact of infections caused by bacteria that produce ESBLs. This could be due to certain factors that increase the risk of the emergence of these strains, such as overcrowded hospitals, increased self-medication, and greater usage of nonprescription antimicrobial drugs, which are more common in low-income countries13,14,15.
Infections from ESBL-producing GNB have been increasing dramatically since a study in Sweden showed that the prevalence of ESBL-producing GNB increased from 1.9% in 2008 to 5% in 2010 in their study population. Another 5-year study between 2005 and 2009 demonstrated that the prevalence increased from 5.2% in 2005 to 13.5% in 200915,16. A Centers for Disease Control and Prevention (CDC) report in 2019 showed a 50% increase in hospital- and community-acquired infections of ESBL-producing GNB between 2012 and 201717.
ESBL producers are often resistant to various categories of antibiotics, such as fluoroquinolones and trimethoprim-sulfamethoxazole (TMP/SMX), in addition to the various agents among the β-lactam classes of antibiotics. Although the use of carbapenems is the preferred treatment for severe infections caused by ESBL-producing organisms, there have been reports of carbapenem-resistant infections18,19.
Carbapenem-resistant Enterobacterales (CRE) produce carbapenemase and are resistant to meropenem, ertapenem, and imipenem20. The first report of CRE was in the early 1990s, and since then, CRE has been disseminated globally21. According to the CDC, CREs are recognized as an ‘urgent threat’ to public health since the mortality rate of hospitalized patients infected with CRE is up to 50%22. In addition, a study concluded that CRE-infected patients are three times more likely to die than infected patients due to carbapenem-susceptible organisms23. Gram-negative bacteria that produce ESBLs and display carbapenem resistance significantly impact patient outcomes, including illness severity and mortality, and are therefore a global healthcare concern. These microorganisms exhibit decreased antibiotic susceptibility, posing treatment challenges. According to a review of 163 studies from low- and middle-income countries that studied antibiotic resistance in hospital-acquired ESKAPE-E (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp., and Escherichia coli) infections, the pooled resistance percentage of hospital-acquired ESKAPE-E infections to essential antibiotics ranged from 16.6 to 85.5% and is generally considered high. Additionally, in this systematic review, the proportions of carbapenem-resistant Gram-negative microorganisms were high: 16.6% in E. coli and 39% in K. pneumoniae. The resistance to third-generation cephalosporins was even greater, as 77% of K. pneumoniae and 75% of E. coli were resistant to this group of antibiotics24.
The aim of this study was to assess the prevalence of infections caused by ESBL-producing and carbapenem-resistant Gram-negative bacilli in patients hospitalized at An-Najah National University Hospital in Nablus, Palestine, and to provide healthcare workers with valuable information on the treatment of these infections. This study is extremely important because multidrug-resistant Gram-negative pathogens are highly drug resistant and pose a threat to public health.
Methods
Study design and setting
A retrospective cross-sectional study was conducted from January to December 2021 at Najah National University Hospital (NNUH), Nablus, to investigate the prevalence of infections caused by ESBL-producing Gram-negative bacteria in patients treated for various diseases. NNUH is a nonprofit academic and tertiary care hospital. The hospital contains 135 beds distributed in different wards and departments (surgical, medical, pediatric, and cardiac departments, in addition to their corresponding intensive care units (ICUs), bone marrow transplant, vascular surgery, and emergency departments). The hospital is considered the main cancer center in the northern governorates of Palestine, where patients from all over the West Bank and Gaza Strip are referred to the NNUH.
Study population
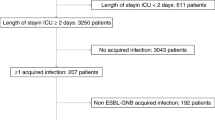
The study included all patients admitted to the NNUH between January 2021 and December 2021 from whom carbapenem-resistant and ESBL-producing E. coli and K. pneumoniae were isolated. All patients who developed hospital-acquired infections (which occurred 48 h after admission) caused by these pathogens were included in the study. We excluded patients who had these pathogens on admission or within 48 h of admission.
Data collection
The hospital information system was used to obtain demographic and medical data, while the hospital's microbiology laboratory provided information on the source of the specimen, the type of microorganism, and the antibiotic susceptibility.
Microbiological methods
Identification and susceptibility testing were performed per the Clinical and Laboratory Standards Institute guidelines using the automated VITEK® 2 Compact system (bio-Mérieux). Identification was performed using VITEK®2 GN ID cards. AST-N204 cards were used to test for antimicrobial agents against aerobic Gram-negative bacilli, including amoxicillin/clavulanic acid, piperacillin, piperacillin-tazobactam, amikacin, imipenem, meropenem, ertapenem, ceftazidime, gentamycin, nitrofurantoin, cefepime, cefotaxime, sulfamethoxazole–trimethoprim, ampicillin and ciprofloxacin25,26. Extended-spectrum β-lactamase production was determined using the ESBL test impeded by the AST-N204 card.
Statistical analysis
The data were entered and analyzed using the Statistical Package for Social Sciences (IBM-SPSS) software, version 21. Continuous variables are displayed as the mean ± standard deviation (SD) or median (interquartile range), while categorical variables are expressed as frequencies and percentages. For categorical variables, the analysis employed either the chi-square test or Fisher’s exact test, as appropriate. All the statistical assessments were two-tailed, with statistical significance defined as a p value less than 0.05, unless explicitly stated otherwise. The reported p values were rounded to three decimal places.
Ethics approval and consent to participate
The Institutional Review Board (IRB) of An-Najah National University approved all aspects of the study protocol, including access to and utilization of patient clinical information. The data collected were exclusively used for clinical research, kept confidential, and not utilized for any other purpose. The project staff had limited access to the collected data, and patient information and hospital names were coded with numbers to ensure anonymity. Since retrospective data were used, the IRB waived the need for informed consent. The study methods adhered to applicable guidelines and regulations.
Results
Demographics and clinical profile of the study population
A total of 188 patients were included in our study. Of these, 91 (48.4%) were male, and 97 (51.6%) were female. The patients' median age (IQR) was 53 (32–66).
Nearly half of the patients had a urinary catheter inserted before the infection (50.9%). Thirty-one patients (18.3%) had a central line, and 49 (28.5%) were intubated for more than 48 h before revealing the presence of the pathogen. Approximately 77.8% of the patients had previous hospitalization within the last three months, and 54.2% had a history of prior antibiotic use within the last three months. Among those with comorbid illnesses, 47.1% had malignancies, and 28% had diabetes mellitus. Hypertensive patients composed 35.3% of the patients, 8.8% were on hemodialysis, and 85 patients (49.4%) were on immunosuppressants. The details can be found in Table 1.
Hospital wards where infections caused by ESBL-producing or CR Gram-negative bacilli occurred in patients
There were 188 patients with infections caused by ESBL-producing or CR bacteria scattered across different hospital wards. The general surgical ward had the highest number of infections (58; 30.9%), followed by the surgical ICU (23; 12.2%). Numerous infections were also reported in patients treated in the internal medicine department (9.6%), the vascular surgery department (9.0%), the medical ICU (8.0%), the cardiac care unit (CCU) (3.7%) and the pediatric ward (2.7%) (Table 2).
Sources of bacteria isolated to produce ESBLs or that exhibit resistance to carbapenems
The greatest number of ESBL-producing or carbapenem-resistant Gram-negative bacilli were isolated from urine (44.7%), followed by from wounds (21.3%), the bloodstream (7.4%), tissues (6.9%), fluids (6.4%) and sputum samples (5.9%), as shown in Table 3.
ESBL-producing E. coli was most frequently isolated from patients (62.8%), while the percentage of ESBL-producing K. pneumoniae was 18.6%. Resistance to carbapenems was found in 11.2% of K. pneumoniae and 7.4% of E. coli, as illustrated in Table 4.
Antibiotic resistance of ESBL-producing and CR-GNB strains isolated during the study period
Most ESBL-producing Gram-negative bacilli were resistant to ampicillin, cefotaxime, ceftazidime, and cefepime. Among the β-lactamase inhibitor-containing antibiotics, 50.4% of the ESBL-producing E. coli were resistant to amoxicillin/clavulanate, while 20.3% were resistant to piperacillin/tazobactam. On the other hand, 64.7% of the ESBL-producing K. pneumoniae isolates were resistant to amoxicillin/clavulanate, whereas 28.6% were resistant to piperacillin/tazobactam. In contrast to ESBL-producing E. coli, for which only 8.1% were resistant to nitrofurantoin, ESBL-producing K. pneumoniae was completely resistant to nitrofurantoin (100%). For gentamicin and amikacin, CR-K. pneumoniae was more resistant (61.9% and 71.4%, respectively) than was CR-E. coli (57.1% and 14.3%, respectively). E. coli and K. pneumoniae strains that were resistant to carbapenems also exhibited high resistance to ciprofloxacin (100% and 90.5%, respectively). For ESBL-producing and carbapenem-resistant strains, resistance to trimethoprim/sulfamethoxazole ranged from 74.3% to 78.6%. All these details are shown in Table 5.
Antibiotics administered empirically to treat infections caused by ESBL-producing isolates or carbapenem-resistant isolates
The vast majority of empirical antibiotic therapies are used in combination regimens. Piperacillin-tazobactam was the most commonly used antibiotic in 21.8% of the patients, followed by amikacin in 18.4%. The empirical use of ceftriaxone and ceftazidime was observed in 13.8% and 9.6%, respectively, of patients. Colistin was used empirically in 6.4% of patients, and ciprofloxacin was administered in 9.6% of patients, as shown in Table 6.
The presence in different clinical materials of E. coli and K. pneumoniae producing ESBLs or showing resistance to carbapenems
Analysing various clinical materials as a source of resistant isolates, it was found that ESBL-producing E. coli were isolated from blood significantly more frequent (p = 0.000). Whereas CR-K. pneumoniae was isolated significantly more frequent from endotracheal aspirates (p = 0.001). Details can be found in Table 7.
Discussion
Infections caused by drug-resistant GNB, mainly ESBL-producing and carbapenem-resistant pathogens, are becoming a major health threat and their number is increasing dramatically worldwide. These are especially infections caused by E. coli and K. pneumoniaee16,27. Additionally, antibiotic resistance is becoming a global concern due to the overuse and misuse of antibiotics and the lack of antibiotic stewardship programs in hospitals. In addition to increasing morbidity and mortality, infections with resistant organisms lead to increased length of hospital stay28,29,30.
A total of 188 patients were included in our study, 51.6% were female patients and the median age of patients was 53 years. This was similar to other studies that showed female predominance of 65% to 35% in males regarding ESBL- producing E. coli infection31, similar to another study that also showed female predominance (57%), and the median age was 70 years32. Another study in Pennsylvania, USA, revealed the predominance of male gender with 51.5% and a median age of 5433.
An Indian study showed that ESBL- producing E.coli were most commonly found in urine (40%) followed by pus samples in 22% of cases31, which is consistent with our study and other studies which found that urine was a major source of bacterial isolates that produce ESBLs33,34. The most statistically significant source of CR K. pneumnieae was the sputum of patients intubated endotracheally (endotracheal aspirates), which is confirmed by another study35
In our study, the surgical ward and surgical ICU had more cases of Gram-negative bacilli producing ESBLs or showing resistance to carbapenems than the medical ward and medical ICU, which is consistent with another study in New Delhi that showed that 55.8% of infections caused by ESBL-producing E. coli were in the surgical ward compared to only 28.6% in the medical ward. In the same study, the similar results were found for ESBL- producing K. pneumonia31. A study in Saudi Arabia also showed that organisms producing ESBL were most commonly found in the surgical ward36 in contrast to that the ICU units had the highest proportion (79%) of isolates in another study in New Delhi37.
One of the important results of our study was that approximately half of the patients had prior antibiotic exposure before the culture result showing growth of E. coli and K. pneumonia producing ESBLs or showing resistance to carbapenems. This was consistent with many studies that concluded that previous antibiotic use, mainly third-generation cephalosporins, increases the risk of developing ESBL- producing GNB16,38,39,40,41. For instance, a study in South Korea showed that antibiotic exposure within 30 days increases the risk of ESBL-producing GNB infection42. This is consistent with our study showing that 54.2% of the patients had previous antibiotic exposure.
Prior or simultaneous colonization with resistant organisms such as ESBL—producing bacteria was a significant risk factor for the development of future infection in many studies16. A French study found prior colonization was the main risk factor for subsequent infections43. In our study, 13% of the screened patients were found to be colonized with ESBL –producing GNB before the onset of infection. This highlights that routine screening for colonization with ESBL –producing GNB may have clinical implications; it may allow the identification of patients at the highest risk of ESBL—producing GNB infections and facilitate the correct allocation of empiric treatment regimens44,45.
We found that approximately 77% of our cases with ESBL –producing GNB were hospitalized within the last three months, in alignment with many other studies showing an association between prior hospitalization and possible ESBL –producing GNB infection41,46,47.
A study conducted in India revealed that 58% of ESBL-producing GNBs had a Foley catheter inserted31. This finding is similar to that of our study, in which 50.9% of our patients had a Foley catheter inserted. This finding was also similar to that of another study in Pennsylvania, in which 55% of the patients had a urinary catheter inserted33. Although 18.3% of our patients had a central line, other studies showed different results; one of those studies showed that only 1.6% of patients had a central venous catheter inserted31. In contrast, another study showed that 58% of HELLP syndrome patients had a central venous catheter inserted33 and that patients with ESBL-producing K. pneumoniae bacteremia were more likely to have invasive devices (urinary and vascular catheters)48. In addition, 43% of the patients developed infection 48 h after central venous catheter insertion in a study performed in Missouri in 201849.
Regarding comorbidities, our study showed that 28% of the patients had diabetes mellitus (DM), which is consistent with the findings of a study conducted in Missouri in 2018 in which 29.4% of the 177 patients had DM49 and 36% had DM in another study33. Similarly, a retrospective study in Missouri showed that 6.8% of patients had end-stage renal disease (ESRD), 15% had end-stage liver disease (ESLD), 19.8% had hematological malignancy, and 20.6% had solid organ malignancy49, which is similar and consistent with the findings of our study; malignancy was found in 47.1% of patients, and ESRD was found in 8.8% of patients. Other studies have shown that 12% of cases are malignant32,40.
Antibiotic use should be based on local antimicrobial surveillance data and should differ according to the severity of infection, type of infection, source of infection and comorbidities of the patients43. A study in Thailand showed that all ESBL-producing K. pneumoniae strains (100%) are resistant to ceftazidime and ampicillin and 16.8% are resistant to amikacin50. Another study demonstrated high resistance to cephalosporins, fluoroquinolones, and penicillin31. These results are similar to those of a previous study conducted in the hematology department of our hospital, which showed 100% resistance to cephalosporins and ampicillin, 75% resistance to levofloxacin, and 62% resistance to TMP-SMX51. Another study in India showed similar results; among the E. coli isolates, 94% were susceptible in vitro to ertapenem, 96% to imipenem, and 76% to piperacillin-tazobactam. The corresponding percentages for ESBL-producing K. pneumoniae isolates were 80%, 84%, and 59%, respectively52. These results are similar to our study, which showed high resistance to cephalosporins and less resistance to carbapenems and amikacin. Additionally, all the ESBL-producing isolates in a Palestinian study had MDR patterns and were resistant to the majority of the β-lactam and non-β-lactam antimicrobial agents. Except for carbapenem, these isolates demonstrated minimal susceptibility to the majority of antimicrobial drugs examined. Carbapenem susceptibility has been associated with β-lactam resistance due to ESBL production; therefore, carbapenems are the drug of choice for infections caused by ESBL-producing pathogens53. The resistance rate to gentamicin in ESBL-producing pathogens isolated from urine culture ranged from 42.9% to 54.2% in our study, whereas it was 81.8% in another Palestinian study54. With regard to imipenem resistance, all the ESBL-producing pathogens in our study were sensitive to this agent, in contrast to the 20% resistance rate to imipenem among ESBL-producing isolates in a Palestinian study conducted by Tayh et al.55. This might be because patients in hospitals with a high resistance rate are frequently given carbapenems, which may have a role in developing multidrug-resistant bacteria. Carbapenem resistance can be attributed to various mechanisms, such as outer membrane proteins (OMPs) or the production of carbapenemases, extended-spectrum β-lactamases and AmpC plasmid enzymes56. Nonetheless, this institution lacks the molecular testing machines needed to explore the molecular mechanism for carbapenem resistance in clinical Enterobacteriaceae isolates, for which we were not able to report the exact resistant strains.
Antibiotic stewardship programmes (ASPs) are necessary to minimize the growing threat of antibiotic resistance. Antibiotic stewardship programmes have shown a decrease in the irrational use of antibiotics, thus decreasing the emergence of multidrug-resistant organisms such as ESBL-producing and CR pathogens. This finding is consistent with a study showing a positive impact of ASP on decreasing the incidence of multidrug-resistant organisms, in which the application of ASP led to a decrease in the incidence of ESBL-producing E. coli and led to a decrease in the irrational use of ciprofloxacin and cephalosporins57.
This research represents a pioneering effort in Palestine to assess the prevalence of infections caused by ESBL-producing and carbapenem-resistant Gram-negative bacilli in patients hospitalized at An-Najah National University Hospital in Nablus, Palestine. The study aims to furnish healthcare professionals with crucial insights into the effective management of such infections. However, our study has several limitations. First, this was a descriptive retrospective study in a single-center hospital; therefore, data for species other than E. coli or K. pneumnieae were missing. Third, we did not assess the change in antibiotic resistance throughout the year or annually thereafter.
Conclusions
Our retrospective analysis provides insight into the frequency and clinical features of healthcare-acquired infections caused by multidrug-resistant GNB, with a specific focus on strains that produce ESBLs and are resistant to carbapenem. This investigation revealed a significant increase in the occurrence of ESBL-producing GNB infections at our institution, reflecting the patterns observed worldwide. The demographic and clinical characteristics of the afflicted patients highlighted the susceptibility of those who had previously been hospitalized, had comorbidities, or a history of antibiotic use. Significantly, the preponderance of infections transpired in the general surgery ward, underscoring the importance of implementing infection control protocols throughout diverse hospital departments. Microbiological research revealed that ESBL-producing E. coli were the most prevalent, with urinary tract infections being the primary source of isolation. The reported resistance patterns, especially the elevated rates of resistance to frequently employed antibiotics, provide a significant problem in the empirical treatment of these illnesses. The increase in carbapenem-resistant bacteria adds complexity to treatment choices, as carbapenem resistance is acknowledged as a substantial public health concern by the CDC. However, further research on the risk factors, causes, associations, and antibiotic susceptibility is needed to determine how to reduce the incidence, mortality and morbidity of this disease.
Data availability
The data from our surveillance are not available in the public domain due to privacy and ethical restrictions, but anyone interested in using the data for scientific purposes is free to request permission from the corresponding authors.
Abbreviations
- CCU:
-
Cardiac care unit
- CDC:
-
Centers for Disease Control and Prevention
- CR:
-
Carbapenem-resistant
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- DM:
-
Diabetes mellitus
- ESBL:
-
Extended-spectrum β-lactamase
- ESLD:
-
End-stage liver disease
- ESRD:
-
End-stage renal disease
- GNB:
-
Gram-negative bacteria
- HIS:
-
Hospital information system
- ICU:
-
Intensive care unit
- IRB:
-
Institutional Review Board
- NNUH:
-
An-Najah National University Hospital
- SICU:
-
Surgical intensive care unit
- TMP/SMX:
-
Trimethoprim-sulfamethoxazole
References
Dhingra, S. et al. Microbial resistance movements: An overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front Public Health 8, 535668 (2020).
Bebell, L. M. & Muiru, A. N. Antibiotic use and emerging resistance: How can resource-limited countries turn the tide?. Glob Heart 9(3), 347–358 (2014).
Shaikh, S., Fatima, J., Shakil, S., Rizvi, S. M. & Kamal, M. A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci 22(1), 90–101 (2015).
Economou, V. & Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 8, 49–61 (2015).
Avershina, E., Shapovalova, V. & Shipulin, G. Fighting antibiotic resistance in hospital-acquired infections: Current state and emerging technologies in disease prevention diagnostics and therapy. Front Microbiol 12, 707330 (2021).
Akram, F., Imtiaz, M. & Haq, I. U. Emergent crisis of antibiotic resistance: A silent pandemic threat to 21(st) century. Microb Pathog 174, 105923 (2023).
Hernando-Amado, S., Laborda, P. & Martinez, J. L. Tackling antibiotic resistance by inducing transient and robust collateral sensitivity. Nat Commun 14(1), 1723 (2023).
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399(10325), 629–655 (2022).
Castanheira, M., Simner, P. J., Bradford, P. A. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 3(3):dlab092 (2021).
Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399(10325):629–655 (2022).
Paterson, D. L. & Bonomo, R. A. Extended-spectrum beta-lactamases: A clinical update. Clin Microbiol Rev 18(4), 657–686 (2005).
Hyun, M., Lee, J. Y., Kim, H. A. & Ryu, S. Y. Comparison of Escherichia coli and Klebsiella pneumoniae acute pyelonephritis in Korean Patients. Infect Chemother 51(2), 130–141 (2019).
Abayneh, M. & Worku, T. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacilli: A meta-analysis report in Ethiopia. Drug Target Insights 14, 16–25 (2020).
Ghafourian, S., Sadeghifard, N., Soheili, S. & Sekawi, Z. Extended spectrum beta-lactamases: Definition, classification and epidemiology. Curr Issues Mol Biol 17, 11–21 (2015).
Strömdahl, H. et al. Prevalence of faecal ESBL carriage in the community and in a hospital setting in a county of Southern Sweden. Eur J Clin Microbiol Infect Dis 30(10), 1159–1162 (2011).
Denis, B. et al. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: A five-year study. Int J Infect Dis 39, 1–6 (2015).
Centers for Disease Control and Prevention. Antimicrobial Resistance: 2019 AR Threats Report. 2021. https://www.cdc.gov/drugresistance/biggest-threats.html. Accessed 3 March 2023.
Morosini, M. I. et al. Antibiotic coresistance in extended-spectrum-beta-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob Agents Chemother 50(8), 2695–2699 (2006).
Rawat, D. & Nair, D. Extended-spectrum β-lactamases in Gram Negative Bacteria. J Glob Infect Dis 2(3), 263–274 (2010).
Chea, N. et al. Improved phenotype-based definition for identifying carbapenemase producers among carbapenem-resistant enterobacteriaceae. Emerg Infect Dis 21(9), 1611–1616 (2015).
Lutgring, J. D. Carbapenem-resistant Enterobacteriaceae: An emerging bacterial threat. Semin Diagn Pathol 36(3), 182–186 (2019).
Centers for Disease Control and Prevention. Healthcare Facilities: Information about CRE. 2019. https://www.cdc.gov/hai/organisms/cre/cre-facilities.html. Accessed 3 March 2023.
Martin, A., Fahrbach, K., Zhao, Q., Lodise, T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to enterobacteriaceae: Results of a systematic literature review and meta-analysis. Open Forum Infect. Dis. 5(7):ofy150 (2018).
Ayobami, O., Brinkwirth, S., Eckmanns, T. & Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: a systematic review and meta-analysis. Emerg Microbes Infect 11(1), 443–451 (2022).
Weinstein MP, Lewis JS, 2nd: The Clinical and Laboratory Standards Institute subcommittee on antimicrobial susceptibility testing: Background, organization, functions, and processes. J Clin Microbiol 2020, 58(3).
Clinical and Laboratory Standards Institute: M100: Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. Wayne, Pennsylvania, USA: Clinical and Laboratory Standards Institute; 2020.
Doi, Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis. 69(Suppl 7):S565–s575 (2019).
Sweileh, W. M. et al. Bibliometric analysis of worldwide publications on multi-, extensively, and totally drug - resistant tuberculosis (2006–2015). Multidiscip Respir Med 11(1), 45 (2016).
Sweileh, W. M. Bibliometric analysis of peer-reviewed literature on antimicrobial stewardship from 1990 to 2019. Global Health 17(1), 1 (2021).
Medina, E., Pieper, D. H. Tackling threats and future problems of multidrug-resistant bacteria. How to overcome the antibiotic crisis: facts, challenges, technologies and future perspectives 3–33 (2016).
Kumar M, Dutta R, Saxena S, Singhal S. Risk factor analysis in clinical isolates of ESBL and MBL (Including NDM-1) producing Escherichia coli and Klebsiella Species in a Tertiary Care Hospital. J. Clin. Diagn. Res. 9(11):Dc08-13 (2015).
Rodríguez-Baño, J. et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol 42(3), 1089–1094 (2004).
Lautenbach, E., Patel, J. B., Bilker, W. B., Edelstein, P. H. & Fishman, N. O. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32(8), 1162–1171 (2001).
Batchoun, R. G., Swedan, S. F. & Shurman, A. M. Extended spectrum beta-lactamases among gram-negative bacterial isolates from clinical specimens in three major hospitals in Northern Jordan. Int J Microbiol 2009, 513874 (2009).
Chen, D. et al. Characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary hospital in Fuzhou, China. J Appl Microbiol 129(5), 1220–1226 (2020).
Kader, A. A. & Kumar, A. K. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med J 25(5), 570–574 (2004).
Mathur, P., Kapil, A., Das, B. & Dhawan, B. Prevalence of extended spectrum beta lactamase producing gram negative bacteria in a tertiary care hospital. Indian J Med Res 115, 153–157 (2002).
Mendelson, G. et al. Prevalence and risk factors of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an Israeli long-term care facility. Eur J Clin Microbiol Infect Dis 24(1), 17–22 (2005).
Tacconelli, E. et al. Estimating the association between antibiotic exposure and colonization with extended-spectrum β-lactamase-producing Gram-negative bacteria using machine learning methods: A multicentre, prospective cohort study. Clin Microbiol Infect 26(1), 87–94 (2020).
Moremi, N. et al. Extended-spectrum beta-lactamase bla(CTX-M-1) group in gram-negative bacteria colonizing patients admitted at Mazimbu hospital and Morogoro Regional hospital in Morogoro, Tanzania. BMC Res Notes 14(1), 77 (2021).
Al-Assil, B., Mahfoud, M. & Hamzeh, A. R. Resistance trends and risk factors of extended spectrum β-lactamases in Escherichia coli infections in Aleppo, Syria. Am J Infect Control 41(7), 597–600 (2013).
Lee, D. S., Lee, C. B. & Lee, S. J. Prevalence and risk factors for extended spectrum Beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol 51(7), 492–497 (2010).
Massart, N. et al. Incidence and risk factors for acquired colonization and infection due to extended-spectrum beta-lactamase-producing Gram-negative bacilli: A retrospective analysis in three ICUs with low multidrug resistance rate. Eur J Clin Microbiol Infect Dis 39(5), 889–895 (2020).
Emmanuel Martinez, A. et al. ESBL-colonization at ICU admission: Impact on subsequent infection, carbapenem-consumption, and outcome. Infect Control Hosp Epidemiol 40(4), 408–413 (2019).
Reddy, P. et al. Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis 45(7), 846–852 (2007).
Tseng, W. P., Chen, Y. C., Chen, S. Y., Chen, S. Y. & Chang, S. C. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob Resist Infect Control 7, 93 (2018).
Guillamet, C. V. & Kollef, M. H. Does this patient have…" "Is this patient at risk for infection with multidrug resistant bacteria?. Intensive Care Med 43(3), 436–439 (2017).
Panhotra, B. R., Saxena, A. K., Al-Ghamdi, A. M. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae hospital acquired bacteremia. Risk factors and clinical outcome. Saudi Med J. 25(12):1871–1876 (2004).
Patolia, S., Abate, G., Patel, N., Patolia, S. & Frey, S. Risk factors and outcomes for multidrug-resistant Gram-negative bacilli bacteremia. Ther Adv Infect Dis 5(1), 11–18 (2018).
Jitsurong, S. & Yodsawat, J. Prevalence of extended-spectrum beta-lactamases (ESBLs) produced in blood isolates of gram-negative bacteria in a teaching hospital in southern Thailand. Southeast Asian J Trop Med Public Health 37(1), 131–135 (2006).
Arman, G. et al. Frequency of microbial isolates and pattern of antimicrobial resistance in patients with hematological malignancies: A cross-sectional study from Palestine. BMC Infect Dis 22(1), 146 (2022).
Chaudhuri, B. N. et al. Incidence of ESBL producers amongst Gram-negative bacilli isolated from intra-abdominal infections across India (based on SMART study, 2007 data). J Assoc Physicians India 59, 287–292 (2011).
Tayh, G. et al. First report of extended-spectrum β-lactamases among clinical isolates of Escherichia coli in Gaza Strip, Palestine. J Glob Antimicrob Resist 6, 17–21 (2016).
Astal, Z., Sharif, F. A., Abdallah, S. A. & Fahd, M. I. Extended spectrum beta-lactamases in Eschericia coli isolated from community-acquired urinary tract infections in the Gaza Strip, Palestine. Ann Saudi Med 24(1), 55–57 (2004).
Tayh, G. et al. Extended-spectrum beta-Lactamases among Enterobacteriaceae isolated from urinary tract infections in Gaza strip, Palestine. Biomed Res Int 2019, 4041801 (2019).
Ye, Y. et al. Mechanism for carbapenem resistance of clinical Enterobacteriaceae isolates. Exp Ther Med 15(1), 1143–1149 (2018).
Peñalva, G. et al. Long-term impact of an educational antimicrobial stewardship programme in primary care on infections caused by extended-spectrum β-lactamase-producing Escherichia coli in the community: An interrupted time-series analysis. Lancet Infect Dis 20(2), 199–207 (2020).
Acknowledgements
The authors thank An-Najah National University and An-Najah National University Hospital for providing an opportunity to carry out this study.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by M.N. and M.A., who also collected the data and performed the analysis. S.A., A.S., and B.A. offered logistical support, designed the study, and assisted in producing the final version of the manuscript. Moreover, S.H.Z., A.A., B.A., and M.Q. conceptualized and designed the study, analyzed and coordinated the data, organized and supervised the field study, critically reviewed the manuscript, interpreted the results, and contributed to writing the final version. Finally, all the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aiesh, B.M., Natsheh, M., Amar, M. et al. Epidemiology and clinical characteristics of patients with healthcare-acquired multidrug-resistant Gram-negative bacilli: a retrospective study from a tertiary care hospital. Sci Rep 14, 3022 (2024). https://doi.org/10.1038/s41598-024-53596-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53596-x
- Springer Nature Limited