Abstract
The Asian tiger mosquito, Aedes albopictus, is an important vector for the transmission of arboviruses such as dengue virus (DENV). Adenosine deaminase (ADA) is a well-characterized metabolic enzyme involved in facilitating blood feeding and (or) arbovirus transmission in some hematophagous insect species. We previously reported the immunologic function of ADA by investigating its effect on mast cell activation and the interaction with mast cell tryptase and chymase. The 2-D gel electrophoresis and mass spectrometry analysis in the current study revealed that ADA is present and upregulated following mosquito blood feeding, as confirmed by qRT-PCR and western blot. In addition, the recombinant ADA efficiently converted adenosine to inosine. Challenging the Raw264.7 and THP-1 cells with recombinant ADA resulted in the upregulation of IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15. The current study further identified recombinant ADA as a positive regulator in NF-κB signaling targeting TAK1. It was also found that recombinant Ae. albopictus ADA facilitates the replication of DENV-2. Compared with cells infected by DENV-2 alone, the co-incubation of recombinant ADA with DENV-2 substantially increased IL-1β, IL-6, TNF-α, and CCL2 gene transcripts in Raw264.7 and THP-1 cells. However, the expression of IFN-β and ISG15 were markedly downregulated in Raw264.7 cells but upregulated in THP-1 cells. These findings suggest that the immunomodulatory protein, Ae. albopictus ADA is involved in mosquito blood feeding and may modulate DENV transmission via macrophage or monocyte-driven immune response.
Similar content being viewed by others
Introduction
Arthropod mosquito is a significant human pathogen vector as diseases caused by mosquito-borne viruses have a significant negative impact on global health. Dengue virus (DENV) is the most prevalent mosquito-borne virus, affecting approximately half of the global population living in dengue-endemic countries. DENV infects 390 million people a year worldwide1. Aedes aegypti is known as the primary vector for DENV transmission. The role of Ae. albopictus in causing DENV outbreaks has grown bigger due to rapid changes in their distribution, posing a profound threat to public health and burdening the global economy2,3,4. DENV is transmitted into vertebrates via the saliva of infected mosquitoes during blood feeding. Numerous studies have reported that mosquito saliva contains a complex and diverse mixture of anti-hemostatic, anti-inflammatory, and immunomodulatory compounds that facilitate blood meal acquisition and arbovirus transmission5,6.
Mosquito saliva contains proteins that are immunogenic to humans, sometimes eliciting severe allergic responses7,8. By injecting a mixture of pharmacologically active proteins into the host during blood-feeding, mosquitos can cause type I hypersensitivity and induce strong immune responses involving macrophages and monocytes9,10. Immunogenic roles of salivary proteins derived from Ae. albopictus was first screened in 201311. However, current studies have only partially elucidated the functions of these proteins. AlboD7L1 reportedly exerts anti-hemostatic and anti-inflammatory effects by inhibiting platelet aggregation and neutrophil recruitment in the host during mosquito blood feeding12. Meanwhile, 34k2 was found to have the ability to induce the production of IgG antibodies, a promising candidate marker for detecting human exposure to Ae. albopictus13,14. This protein may also be involved in mast cell-driven immune responses following mosquito biting, as demonstrated by its interaction with mast cells, leading to the release of specific proteases tryptase, and chymase15.
When it comes to the effect of mosquito saliva on DENV, many researchers have proved the role of proteins in the saliva in DENV replication. In Ae. aegypti models infected with DENV, the virus titer, and systemic infection were magnified by mosquito saliva16,17,18. Several salivary components have been identified as the factors affecting the infection or transmission of arboviruses. A saliva protein of Ae. aegypti called AsSG34 reportedly enhanced the transmission of DENV to the mammalian host19. AaVA-1, a saliva protein of Ae. aegypti reportedly induced the enhancement of DENV replication in macrophages20. On the contrary, a saliva protein of Ae. aegypti called D7 was reported to inhibit DENV infection21. Although studies have associated Ae. albopictus with the transmission of DENV22,23,24, very few investigated the effect of salivary proteins on DENV infection in Ae. albopictus model.
The genome sequences of several model organisms and humans reveal the presence of more than one adenosine deaminase (ADA) gene in metazoan25. ADA catalyzes the deamination of adenosine and deoxyadenosine into their respective inosine nucleosides. ADA is an important immune regulatory molecule in humans, which plays an important role in the maturation and maintenance of the immune system. ADA deficiency leads to an accumulation of toxic purine degradation by-products, primarily affecting lymphocytes, leading to severe combined immunodeficiency (SCID) caused by adenosine deaminase deficiency26,27. Higher Diptera carry an unusually high number of ADA genes compared with other organisms25,28. ADA genes of mosquitos were first found in Culex quinquefascia-tus and Ae. aegypti, whereby their activity was measured in the latter28. High expression of ADA was reported in the salivary glands of Ae. aegypti, which may be vital for blood feeding and degrading purinergic mediators of hemostasis and inflammation29. Additionally, evidence has demonstrated the role of ADA in virus replication in Ae. aegypti30,31. However, the underlying mechanisms of ADA are not fully characterized. A significant expression level of ADA gene in salivary glands of female Ae. albopictus was reported, which is currently regarded as a putative secreted salivary protein according to transcriptomic and proteomic analysis32. However, no studies have investigated the activity and function of ADA in Ae. albopictus.
In this work, we screened and identified the upregulated salivary protein, ADA, from the salivary glands of blood meal in female Ae. albopictus model. Studies have revealed the enzymatic activity of ADA of Ae. albopictus, particularly its role as an immunomodulatory factor involved in the inflammatory response. This response was induced by mosquito blood feeding, which triggers monocyte or macrophage-driven immune response, demonstrating a vital role in DENV transmission through modulating the release of associated cytokines.
Results
Ae. albopictus ADA is involved in blood-feeding
Aedes albopictus triggers the host immune system by injecting salivary proteins. To find the key target proteins related to blood feeding, a comparative analysis of salivary protein profiles between female models with and without blood meal was performed using 2-D gel electrophoresis (2-DE) (Fig. 1). Analysis of the gels using 2D Image Platinum software revealed 547 spots in the unfed female salivary gland, mainly distributed between 20 and 130 kDa. Comparisons between the unfed and blood-fed groups revealed 26 protein spots with a saliency of more than two, which were further characterized. Out of these 26 spots, 22 spots were downregulated (Fig. 1A) and 4 spots were upregulated (Fig. 1B) in the blood-fed group compared with the unfed group. The results suggest that these salivary proteins may be involved in mosquito blood-feeding.
Two-dimensional gel analysis of differentially expressed salivary gland proteins in female Ae. albopictus mosquitoes with or without a blood meal. Salivary glands of 300 μg extracted from five-day-old unfed or blood-fed mosquitoes were loaded onto 3–10 NL immobilins (24 cm) before SDS-PAGE separation on a 12.5% precast gel. Protein spots were stained with Coomassie Blue R-250 for 2 h. Molecular weight markers are indicated on the left in kDa. Isoelectric points (pI) are indicated at the top. Gel profiles were compared using Image Master Platinum software. The spots corresponding to the differentially expressed proteins are indicated by arrows (fold change of > 2.0; Anova of < 0.05). (A) 2D-gel separation of salivary proteins extracted from unfed female mosquitoes. (B) 2D-gel separation of salivary proteins extracted from blood-fed female mosquitoes. Data are representative of three independent experiments.
To identify the key salivary gland proteins, 26 variant spots were excised and digested by trypsin and analyzed by MALDI-TOF/TOF mass spectrometry. As shown in Table 1, there were 23 major protein spots, of which ADA exhibited the largest fold expression among these upregulated proteins. However, the other three spots were not detected, possibly due to the high complexity of the spots in the sample. To validate the upregulated ADA in salivary glands of the blood-fed group, its expression in salivary glands of female Ae. albopictus was first confirmed (Fig. 2A) via immunoblotting using an anti-ADA antibody. The results demonstrate the upregulation of ADA in the salivary glands of the blood-fed group following the feeding (Fig. 2B). These results imply the role of ADA in mosquito blood-feeding.
ADA is predominantly present in salivary glands and is upregulated following a mosquito blood meal. (A) Total mRNAs were separately extracted from salivary glands or female mosquitoes with salivary glands removed; the mRNA level of ADA was analyzed by qRT-PCR. (B) Salivary glands were collected from five-day-old female mosquitoes with (BF) or without (UF) blood-fed and the expression of salivary ADA was detected by immunoblotting with anti-ADA antibody (down). The signal intensity of each protein corresponds to the β-actin (up). n = 3. *p < 0.05; **p < 0.01.
Aedes albopictus ADA shows enzymic activity
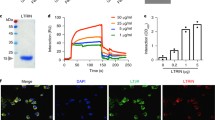
To further examine the functions of ADA, ADA was expressed in prokaryotes and purified as a soluble protein in the supernatant of bacterial lysate. ADA catalyzes the deamination of adenosine to inosine and deoxyadenosine to deoxyinosine. To test the activity of ADA in the saliva of Ae. albopictus, soluble proteins from salivary gland homogenates of Ae. albopictus and the recombinant ADA (rAb-ADA)were incubated in the presence of adenosine. The changes were measured spectrophotometrically at the wavelengths from 220 to 300 nm. The substrate adenosine absorbs light at 265 nm, and the product of ADA activity, inosine, absorbs at 241 nm. Compared to the blank control (Fig. 3A), the salivary homogenate equivalent to 0.2 pairs salivary gland converted adenosine to inosine (Fig. 3B). Similarly, rAb-ADA of Ae. albopictus converted adenosine to inosine (Fig. 3C). The differential spectrum (Fig. 3B right panel and Fig. 3C right panel) shows the decrease of adenosine (265 nm) and the gradual increase of inosine (241 nm) in the presence of salivary gland homogenates or rAb-ADA of Ae. albopictus. These results demonstrate the adenosine deaminase activity in Ae. albopictus, proving the enzymatic activity of rAb-ADA.
Enzymatic activity of Ae. albopictus ADA. ADA activity was measured by the spectrophotometric method. ADA activity in salivary homogenates of adult female Ae. albopictus: a cuvette containing 1 mM adenosine in 100 µL of HS buffer was continuously scanned 30 times at the wavelengths ranging from 230 to 300 nm for samples without (A) or with (B) the addition of 10 μL salivary gland homogenates extracted from 20 pairs of female salivary glands at 5-day-old PE (left panel). The differential spectra (right panel) of the data in the left panel were obtained by subtracting each read from the read at time zero. (C) Recombinant ADA exhibiting adenosine deaminase activity: a cuvette containing 1 mM adenosine in 100 µL of HS buffer was continuously scanned 30 times at the wavelengths ranging from 230 to 300 nm following the addition of 0.67 µM recombinant ADA (left panel). The differential spectra (right panel) of the data in the left panel were obtained by subtracting each read from the read at time zero. Data are representative of three independent experiments. Colors represent different times.
rAb-ADA induces cytokines release from monocytes and macrophages
We previously demonstrated the activation of mast cells by ADA and its interaction with specific proteases in mast cells15. Macrophages reside in the skin dermis, whereas monocytes circulate in the blood, enabling them to directly respond to mosquito saliva proteins. Therefore, we evaluated the immunomodulatory roles of rAb-ADA in Raw264.7 and THP-1 cells by evaluating the levels of related cytokines. As depicted in the results, the expression of genes encoding cytokines, IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15, were significantly upregulated when challenged with rAb-ADA (Figs. 4A, S1). Similarly, the production of IL-1β, IL-6, and TNF-α in supernatants was markedly induced by rAb-ADA (Fig. 4B). These results suggest that rAb-ADA may modulate inflammatory responses following mosquito biting via the activation of macrophage or monocyte.
Recombinant Ae. albopictus ADA promotes cytokines production in vitro. (A) Human THP-1 cells were treated with various doses (0.02, 0.2, and 2 μg/mL) of recombinant Ae. albopictus ADA. RNA was isolated from the cells at various time points (6 h,12 h, and 24 h) following the stimulation, cDNA was generated, and qRT-PCR was performed to measure the mRNA level of IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15. The data were normalized to human actin by applying the ΔΔCT method and are presented as percentages of the average ΔΔCT value of untreated (PBS) cells. (B) ELISA of IL-1β, IL-6, and TNF-α in supernatants of Raw 264.7 cells treated with various doses (0.02, 0.2, and 2 μg/mL) of recombinant Ae. albopictus ADA at 24 h. A nonparametric Mann–Whitney test was applied for the statistical analysis. Labels: ns indicates insignificant (p > 0.05); *p < 0.05; **p < 0.01; ***p < 0.001. The data are representative of three independent experiments.
rAb-ADA activates the NF-κB signaling pathway by targeting TAK1
The nuclear factor, NF-κB, is a prototypical proinflammatory signaling pathway that induces the expression of proinflammatory genes including cytokines, chemokines, etc.33. Therefore, we investigated the regulatory effects of rAb-ADA on the NF-κB pathway by detecting the phosphorylation levels of the p65 and IκBα, as well as the nuclear translocation of p65. Immunoblot assay revealed that rAb-ADA induced the degradation of IκBα and upregulated the phosphorylation of the p65 and IκBα (Fig. 5A). Notably, immunofluorescence analysis confirmed the role of rAb-ADA in promoting the localization of p65 in the nucleus of Raw264.7(Fig. 5B).
Recombinant Ae. albopictus ADA activates the NF-κB pathway by targeting Takinib. (A) Immunoblot analysis of phosphorylated IκBα(p-IκBα), total proteins of IκBα(IκBα), phosphorylated p65(p-p65), and p65 in human THP-1 cells from untreated (Medium) or treated with ADA (2 μg /mL) groups at various time points (15 min, 30 min, and 1 h). (B) Confocal microscopy of p65 in mouse Raw264.7 cells from untreated (Medium) or treated with ADA (2 μg /mL) groups at 1 h. (C) Human THP-1 cells treated with the inhibitors, Takinib (10 μM) or DMSO, for 3 h before being stimulated with ADA (2 μg/mL) at various time points (15 min, 30 min, and 1 h); p-IκBα, IκBα, p-p65and p65 were analyzed via Immunoblot assay. (D) Human THP-1 cells were treated with the inhibitors, Takinib (10 μM) or DMSO, for 3 h before stimulation with ADA (2 μg/mL) for 24 h; RNA was then isolated from the cells, cDNA was generated, and qRT-PCR was performed to measure the mRNA level of IL-1β, IL-6, and TNF-α. A nonparametric Mann–Whitney test was applied for the statistical analysis. *p < 0.05; **p < 0.01. The data are representative of two independent experiments.
Furthermore, we analyzed the effects of NF-κB inhibitor, BAY11–7082, and TAK1 inhibitor, Takinib, on rAb-ADA-mediated NF-κB activation. BAY11–7082, a nuclear factor-κB inhibitor irreversibly inhibits the phosphorylation and degradation of IκBα34. Takinib, a selective TAK1 inhibitor, inhibits TAK1 activation35. TAK1 phosphorylates IKKβ through its proximity to the IKK complex, which leads to NF-κB activation via the phosphorylation and subsequent degradation of IκB proteins36. The results demonstrate a significant downregulation of p-IκBα and p-p65 by Takinib (Fig. 5C) and BAY11–7082 (Fig. S2A) treatment at 1 h. The level of mRNA corresponding to IL-1β, IL-6, and TNF-α was significantly suppressed by Takinib (Fig. 5D), but not by BAY11–7082 (Fig. S2B). In general, the results demonstrate that rAb-ADA activates NF-κB signaling pathway by targeting TAK1.
rAb-ADA promotes DENV-2 replication
Mosquito saliva has been demonstrated to facilitate viral transmission. Therefore, to determine the potential role of ADA in DENV-2 infection, we incubated the DENV-2-infected Raw264.7 cells or THP-1 cells with or without rAb-ADA for 6 h, 12 h, and 24 h. Analysis of the expression of DENV-2 genes revealed that the viral load is higher in 24 h (Fig. S3) and the replication of the virus dose-dependently increased at 24 h in both cell lines (Fig. 6A,B). Further, Raw264.7 cells were stained for dsRNA (J2 antibody) detection, and flow cytometry was used to analyze the J2 fluorescence signal that signifies DENV replication. The results demonstrate the enhanced DENV replication in the presence of rAb-ADA (Fig. 6C). These findings suggest the potential involvement of ADA in the transmission of DENV-2 via facilitating DENV-2 replication.
Effects of recombinant Ae. albopictus ADA on DENV-2 replication. Raw264.7 cells (A) and human THP-1 cells (B) were treated with DENV-2 (MOI = 0.25) alone or in combination with various doses (0.02, 0.2, and 2 μg/mL) of recombinant Ae. albopictus ADA. At various time points (6 h,12 h, and 24 h) following the stimulation, RNA was isolated from the cells, cDNA was generated, and qRT-PCR was performed to detect the genomes of the DENV envelope. Raw 264.7 cells treated with DENV-2 alone or in combination with recombinant Ae. albopictus ADA (2 μg/mL) and untreated group (Medium) were stained for dsRNA detection (J2 antibody); a flow cytometry (C) was applied to analyze the J2 fluorescence signal: representative result obtained from the flow cytometry analysis (left panel) and quantification of mean fluorescence intensity (MFI) (right panel) with n = 3. The data obtained from the analysis of Raw 264.7 cells were normalized to the mouse GAPDH using the ΔΔCT method and are presented as percentages of the average ΔΔCT value of the cells treated with DENV-2 alone; human THP-1 cells were normalized to human actin using the ΔΔCT method and are presented as percentages of the average ΔΔCT value of the cells treated with DENV-2 alone. A nonparametric Mann–Whitney test was applied for the statistical analysis. Labels: ns, insignificant (p > 0.05); *p < 0.05; **p < 0.01; ***p < 0.001. The data are representative of three independent experiments.
rAb-ADA regulates cytokine production during DENV-2 infection
Since DENV-2 induces cytokines production in macrophages and monocytes, it would be interesting to verify the immunomodulatory role of rAb-ADA during DENV-2 infection. Thus, we next determined the expression of cytokine in Raw264.7 and THP-1 cells infected with DENV-2 in the presence or absence of rAb-ADA for 24 h. Compared to infection without rAb-ADA, the co-administration of DENV-2 and rAb-ADA resulted in a substantial increase of inflammatory mediator genes IL-1β, IL-6, TNF-α, and CCL2 in Raw264.7 and THP-1 cells. However, the increment is dose-dependent in Raw264.7 but dose-dependent in THP-1 cells (Fig. 7A,B). The production of IL-1β, IL-6, and TNF-α in supernatant was also remarkably induced by rAb-ADA in DENV-infected cells (Fig. S3). Additionally, mRNA expression of IFN-β and ISG15 exhibited a different trend in Raw264.7 cells compared to the human THP-1 cells (Fig. 7C). The transcription level of IFN-β and ISG15 decreased in Raw264.7 cells incubated with rAb-ADA during DENV-2 infection. On the contrary, the transcription levels of IFN-β and ISG15 were enhanced in human THP-1 cells despite the same treatment conditions as Raw264.7 cells. These results suggest the modulation of cytokines released from DENV-2-infected macrophages and monocytes by ADA.
Recombinant Ae. albopictus ADA in combination with DENV-2 infection induces a substantial increase in the expression of cytokines. qRT-PCR analysis of cytokines (IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15) expression in Raw264.7 and human THP-1 after the infection with DENV-2 (MOI = 0.25) alone or in combination with various doses (0.02, 0.2 or 2 μg/mL) of recombinant Ae. albopictus ADA at 24 h. The data from Raw 264.7 cells were normalized to mouse GAPDH using the ΔΔCT method and are presented as percentages of the average ΔΔCT value of the cells treated with DENV-2 alone; human THP-1 cells were normalized to human actin using the ΔΔCT method and are presented as percentages of the average ΔΔCT value of the cells treated with DENV-2 alone. A nonparametric Mann–Whitney test was applied for the statistical analysis. Labels: ns indicates insignificant (p > 0.05); *p < 0.05; **p < 0.01; ***p < 0.001. The data are representative of three independent experiments.
Discussion
Aedes albopictus is currently ranked among the top 100 invasive species worldwide and can be found on all continents37. During probing and blood feeding, mosquitoes inject their saliva into the skin of vertebrate hosts19. Considering the roles of salivary proteins in inducing strong inflammatory immune responses at the bite sites, and promoting arboviruses transmission during blood feeding38,39, we compared the protein expression profiles in the saliva of blood-fed and unfed female mosquitos. Among the identified salivary proteins, ADA of Ae. albopictus, which can degranulate mast cells, was screened as a promising candidate. Then, the expression of ADA in the salivary glands of Ae. albopictus was verified via western blot. The analysis revealed a significant upregulation of ADA in female salivary glands following blood meal.
ADA is a crucial purine metabolic enzyme involved in the conversion of adenosine and 2′-deoxyadenosine into inosine and 2-deoxyinosine, respectively40. The enzyme activity of ADA has been demonstrated in the saliva of C. quinquefasciatus and Ae. aegypti (mosquitoes)28, Glossina m. morsitans (tsetse fly)41, Lutzomiya longipalpis (sand fly)42. This enzyme may also help blood feeding and regulate the expression of inflammatory cytokines28,43,44. Thus, we cloned and purified the recombinant Ae. albopictus-derived ADA and tested its enzyme activity. The results demonstrate the catalytic activity of ADA in the conversion of adenosine into inosine.
Studies have shown the interaction between viral components and mosquito saliva with the cells within the skin of the hosts, which are the first line of defense. Since these cells are permissive to infection and capable of eliciting immune responses, they play a dual role during the infection45. Therefore, a comprehensive understanding of the interaction between these cells, arbovirus, and mosquito saliva is important for developing strategies to combat mosquito-borne diseases. However, there are only a handful of studies investigating the interactions between certain salivary proteins and these cells46, particularly in Ae. albopictus.
A study reported the immunogenic role of ADA in Ae. albopictus11. Recently, we reported the interaction between ADA derived from the saliva of Ae. albopictus and mast cell-specific proteases including tryptase and chymase. The findings suggest the activation of mast cell-driven immune response by ADA, which leads to inflammation15. Nonetheless, other functions of ADA in Ae. albopictus remain unexplored. Macrophages, the monocyte-lineage cells, are antigen-presenting cells, which have an important role in immune response. To further investigate the potential function of ADA, we stimulated Raw264.7 macrophages and THP-1 monocytes with rAb-ADA. In this study, we identified ADA from Ae. albopictus as an immunomodulatory factor based on the increased levels of TNF-α, IL-6, IL-1β, TNF-α, IL-6, CCL2, IFN-β, and ISG15. Additionally, IL-1β, TNF-α, and IL-6 exerted pleiotropic effects on diverse cell types47. The major function of CCL2 production is to recruit cells48, which are important immune mediators involved in host inflammatory response. Our study further identified rAb-ADA as a positive regulator of NF-κB signaling, which targets TAK1. In general, the results demonstrate the role of rAb-ADA in modulating host inflammatory response via the activation of macrophages by targeting TAK1.
During viral transmission, both mosquito saliva and viruses are injected into the host. Some salivary proteins contribute to virus dissemination in the host by enhancing virus replication in host cells. Studies have found that ADA is highly expressed in the salivary glands of Ae. aegypti and contributes to DENV infection30,31. Another study reported the role of NeSt1 in promoting DENV and ZIKV replication in mice49. The current study revealed that the treatment of infected macrophages and monocyte cells with rAb-ADA leads to a high DENV-2 load. However, the role of salivary proteins in DENV replication and the associated mechanisms remain unclear. Recent studies have associated several key factors with DENV replication. In the infection models using mosquito cells, the double-stranded RNA (dsRNA) binding protein Loquacious, epigenetic regulator DIDO1, and septin 2 were found essential for the replication of DENV or ZIKV50,51,52. This finding sparks interest in determining the possible role of ADA in facilitating DENV-2 replication via modulating those factor-like proteins. The innate antiviral response to DENV is initiated by various immune cells and inflammatory mediators. Macrophages and monocytes are the main targets of DENV infection, whose functions include recognizing pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs)53. Toll-like receptors (TLRs) are the major PRRs that are coupled to detect specific viral components and induce the production of inflammatory cytokines, chemokines, and IFNs54. The effectors' action primarily results in antiviral responses, which could lead to disease pathogenesis should an imbalance in responses occur. In our studies, we observed that the mRNA expression level of IL-1β, IL-6, TNF-α, and CCL-2 was increased in both DENV-2-infected immune cells. Enhanced induction of IFN-β and ISG15 by rAb-ADA during DENV-2 infection was observed in THP-1 cells. Meanwhile, rAb-ADA suppressed the induction of IFN-β and ISG15 in Raw264.7 cells, suggesting the role of ADA in regulating cytokine production during DENV-2 infection. NF-κB is critical for TLR-mediated antiviral IFN responses and pro-inflammatory activation. These findings demonstrate that rAb-ADA is a positive regulator of NF-κB signaling. However, the mechanisms of ADA-altered cytokine expression in facilitating DENV-2 replication via targeting the NF-κB signaling require further investigation.
Conclusions
In this work, distinction protein spots in the salivary gland of female Ae. albopictus were modulated by blood-feeding. The results demonstrate the upregulation of ADA as evidenced in WB. Moreover, enhanced expression of ADA and its enzymatic activity in the salivary gland of female Ae. albopictus was detected. Subsequently, a rAb-ADA protein with ADA enzymatic activity was obtained. Further investigation revealed the induction of proinflammatory cytokines by rAb-ADA via targeting TAK1, facilitating DENV-2 replication in Raw264.7 and THP-1 cells. The results demonstrate the possible role of ADA as an immunomodulatory factor that enhances DENV-2 replication in immune cells. These findings will help establish the role of Ae. albopictus salivary protein in arboviruses transmission, and may provide a theoretical basis for preventing DENV transmission in nature.
Methods
Mosquitoes, cells, virus
Aedes albopictus mosquitoes (Guangzhou strain) were kindly provided by Prof. Zhao Tongyan (Beijing Institute of Microbiology and Epidemiology) and reared as previously described55. Raw264.7 cells were purchased from the Center for Type Culture Collection (Wuhan, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), streptomycin (100 mg/mL), and penicillin (100 U/mL) and maintained at 37 °C (5% CO2). THP-1 cells were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China) and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), streptomycin (100 mg/mL), and penicillin (100U/mL) and maintained at 37 °C (5% CO2). DENV-2 strain was propagated in Ae. albopictus C6/36 cells. The virus titers were determined by a plaque formation assay.
Preparations of Ae. albopictus salivary gland protein extracts
Salivary glands were dissected from adult female mosquitoes on day five post-emergence (PE) of unfed and blood-fed groups. These salivary glands were re-suspended in 100 μL PBS and kept at − 80 °C until further use. Salivary glands were disrupted using a tissue homogenizer (MIULAB, China) for 10 min on ice and were centrifuged at 12,000g for 45 min. The supernatant was kept at − 80 °C until further use.
Two-dimensional gel electrophoresis and MALDI-TOF MS analysis
Salivary proteins were desalted using a 2-D Clean-Up kit (GE Healthcare, USA) and quantified using a Micro BCA Protein Assay Kit (Pierce, Rockford, IL). Proteins of 300 μg were loaded on an Immobiline DryStrip (pI 3–10, 24 cm, Bio-Rad, USA) to perform the first dimension isoelectric focusing separation. Following 14 h of rehydration, the strips were focused using Ettan IPGphor III (GE Healthcare, USA) according to the manufacturer's instructions. The focused strips were then applied to the SDS-PAGE for the second dimension. After the electrophoresis, the gels were stained using Coomassie Brilliant Blue (CBB) and viewed using ImageScanner III (GE Healthcare, USA). The images were analyzed using ImageMaster 2D Platinum 6.0 software (GE Healthcare, Buckinghamshire, UK). The molecular weight (MW), by which the isoelectric point (pI) and normalized volume of each protein spot from unfed or blood-fed gel samples were calculated following the software manuals supplied by the manufacturer. The average normalized volume (ANV) of each major protein in the unfed and blood-fed groups was obtained. The fold expression of ≥ 2 of the major proteins was calculated and compared between the two 2-DE gel images.
Differentially expressed protein spots were selected and excised from the 2-D gels and subjected to in-gel digestion. A sample of 1 μL in volume was spotted onto a MALDI-TOF MS plate with 0.5 μL of a-cyano-4-hydroxycinnamic acid (a-CHCA) for analysis by 4800 Plus MALDI TOF/TOFTM analyzer (Applied Biosystems, USA).
Enzyme assays
Measurement of adenosine deaminase activity was performed in quartz microcuvettes via a spectrophotometric approach. Adenosine of 1 mM in a buffer containing 10 mM Hepes and 150 mM NaCl (HS, pH = 7.0) was added to the cuvette, followed by the addition of 10 μL of salivary gland homogenates extracted from 20 pairs of female salivary glands on day five PE or 0.67 μM purified rAb-ADA expressed in the Escherichia coli BL21 (DE3) strain. After mixing the sample in the solution, the absorbance at 220 and 300 nm was continuously measured 30 times by using a UV-2600 spectrophotometer (Shimadzu, Japan).
Prokaryotic expression and purification of recombinant Ae. albopictus ADA
ADA from Ae. albopictus was expressed and purified as described previously15. A DNA fragment encoding a mature ADA (GenBank: AAV90660.1) was amplified by PCR and inserted into the cloning site of the pET32a(+) vector. E. coli BL21 (DE3) cells were transformed with the recombinant plasmid (pET32a(+)-ADA) and 1 mM isopropyl-β-d-thiogalactosidase (IPTG) was added to the Luria–Bertani (LB) medium to induce rAb-ADA expression. rAb-ADA protein was purified using His Bind Purification Kit (Novagen) according to the manufacturer’s instructions and dialyzed against 1× PBS buffer at 4 °C overnight. The purified protein was analyzed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using Coomassie brilliant blue R-250. The absence of endotoxin was tested using an ELISA Kit for Lipopolysaccharides as reported in Ref15.
Production of rabbit anti-ADA polyclonal antibody
rAb-ADA of 400 μg was mixed with Freund's Complete Adjuvant (Sigma, USA) at a ratio of 1:1. The suspension was subcutaneously injected at various sites on the back of a 15-week-old male New Zealand rabbit during the first immunization. The subsequent subcutaneous injections with a half-dose of rAb-ADA mixed with incomplete Freund’s adjuvant (Sigma, USA) at a ratio of 1:1 was performed every week. Rabbit blood was collected after the fourth immunization and stored at − 20 °C until further use.
Western blotting
Samples were separated on 10% SDS-PAGE and subsequently transferred to the Immobilon-NC Transfer Membrane (Millipore, USA). The membranes were blocked with 5% milk in Tris-buffered saline overnight and subsequently incubated with the following primary antibodies for 2 h at room temperature: anti-p65(8243S, CST); anti-phospho-NF-κB p65 (3033S, CST), anti-IκBα (4812S, CST), anti-p-IκBα (2859S, CST), anti-β-actin (3700S, CST), anti-His (T0009, Affinity), anti-ADA polyclonal. After the incubation, membranes were washed three times and incubated with a secondary HRP conjugated goat anti-rabbit (SA0001-2, proteintech) or goat anti-mouse (RS0001, Immunoway) (at 1:2000 dilution) for 1 h at 37 °C. The signals corresponding to the proteins were detected by enhanced chemiluminescence (ECL) (Millipore, USA). All the blots were cut prior to hybridisation with antibodies.
rAb-ADA-stimulation in THP-1 and Raw264.7 cells
The THP-1 and Raw264.7 cells were seeded at 25,000 cells/well in 24-well plates and maintained at 37 °C (5% CO2) for approximately 12 h until 70% confluence was reached. The THP-1 cells were treated with various doses (0.02, 0.2, and 2 μg/mL) of rAb-ADA. The cells were harvested at different time points (6 h, 12 h, and 24 h) post-stimulation to determine the mRNA expression of IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15 via qRT-PCR. The Raw264.7 cells were treated with various doses (0.02, 0.2, and 2 μg/mL) of recombinant Ae. albopictus ADA was harvested at 24 h, and the supernatants were collected and kept at − 80 °C until further use.
The infection of Raw264.7 and THP-1 cells with DENV-2
The Raw264.7 and THP-1 cells were seeded at 25,000 cells/well in 24-well plates and maintained at 37 °C (5% CO2) for approximately 12 h until 70% confluence was reached. The cells were either treated with DENV-2 0.25 multiplicity of infection (MOI) alone or in combination with various doses (0.02, 0.2, and 2 μg/mL) of rAb-ADA for 6 h,12 h and 24 h. After the incubation, the cells were harvested to determine viral replication and mRNA expression of IL-1β, IL-6, TNF-α, CCL2, IFN-β, and ISG15 via qRT-PCR.
Quantitative real-time PCR
The total RNA was isolated from tissues and cells by using TRIzol reagent (Invitrogen, USA) and reverse-transcribed into cDNA using PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Japan) according to the manufacturer’s protocol. Real-time qRT-PCR was performed on Bio-Rad CFX-96 Connect Real-Time Detection System to measure the mRNA levels of tested genes. Primer sequences are shown in Supplementary Table 1. The 2−∆∆CT method was used to determine relative expression levels, and A nonparametric Mann–Whitney test was used to determine if the differences were statistically significant. All analyses were performed using the GraphPad Prism statistical software (Version8.0.2).
Enzyme-linked immunosorbent assay (ELISA)
IL-1β, IL-6, and TNF-α in supernatants of Raw264.7 mouse macrophage were detected by using mouse IL-1β, IL-6, and TNF-α ELISA kit (Invitrogen) according to the manufacturer’s instructions.
Intracellular staining for flow cytometry
Raw264.7 cells were first fixed for 30 min and further permeabilized for 30 min using eBioscience™ Intracellular fixation & permeabilization buffer set (88-8824-00, Thermo Fisher Scientific, USA). The cells were washed twice with PBS + 1% FBS. Next, the Raw264.7 cells were blocked for 30 min with PBS + 5% FBS, washed twice, and stained with dsRNA using J2 antibody (10010200, Nordic-MUbio) (1:500 dilution) for 1 h, followed by three cycles of washing with PBS + 1% FBS. The cells were then stained with a secondary antibody, Goat anti-Mouse IgG conjugated with PE (12–4010-82, Invitrogen), at 1:1500 dilution for 1 h in PBS + 5% FBS, followed by three cycles of washes. The cells were finally resuspended in PBS + 1% FBS for analysis on Cytoflex LX (Beckman, USA).
Ethical approval
New Zealand rabbit were provided by the Animal Center of Guizhou Medical University and was cultured in a pathogen-free environment. Animal experiments was conducted and approved by the Animal Care welfare Committee of Guizhou Medical University under protocol number 1603140 and all methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Data availability
Data supporting the conclusions of this article are included within the article and its additional files. The raw datasets used and analysed during the present study are available from the corresponding author upon reasonable request.
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507. https://doi.org/10.1038/nature12060 (2013).
Paupy, C., Delatte, H., Bagny, L., Corbel, V. & Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 11, 1177–1185. https://doi.org/10.1016/j.micinf.2009.05.005 (2009).
Lwande, O. W. et al. Globe-trotting aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis. 20, 71–81. https://doi.org/10.1089/vbz.2019.2486 (2020).
Rezza, G. Aedes albopictus and the reemergence of Dengue. BMC Public Health 12, 72. https://doi.org/10.1186/1471-2458-12-72 (2012).
Ribeiro, J. M. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. https://doi.org/10.1146/annurev.en.32.010187.002335 (1987).
Onyango, M. G., Ciota, A. T. & Kramer, L. D. The vector–host–pathogen interface: The next frontier in the battle against mosquito-borne viral diseases?. Front. Cell Infect. Microbiol. 10, 564518. https://doi.org/10.3389/fcimb.2020.564518 (2020).
Cantillo, J. F., Fernandez-Caldas, E. & Puerta, L. Immunological aspects of the immune response induced by mosquito allergens. Int. Arch. Allergy Immunol. 165, 271–282. https://doi.org/10.1159/000371349 (2014).
Depinay, N., Hacini, F., Beghdadi, W., Peronet, R. & Mecheri, S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 176, 4141–4146. https://doi.org/10.4049/jimmunol.176.7.4141 (2006).
Vander Does, A., Labib, A. & Yosipovitch, G. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Front. Immunol. 13, 1024559. https://doi.org/10.3389/fimmu.2022.1024559 (2022).
Guerrero, D. et al. Evaluation of cutaneous immune response in a controlled human in vivo model of mosquito bites. Nat. Commun. 13, 7036. https://doi.org/10.1038/s41467-022-34534-9 (2022).
Doucoure, S. et al. First screening of Aedes albopictus immunogenic salivary proteins. Insect Mol. Biol. 22, 411–423. https://doi.org/10.1111/imb.12032 (2013).
Calvo, E. Aedes albopictus D7 salivary protein prevents host hemostasis and inflammation. Biomolecules https://doi.org/10.3390/biom10101372 (2020).
Buezo Montero, S. et al. IgG antibody responses to the Aedes albopictus 34k2 salivary protein as novel candidate marker of human exposure to the tiger mosquito. Front. Cell Infect. Microbiol. 10, 377. https://doi.org/10.3389/fcimb.2020.00377 (2020).
Buezo Montero, S. et al. Human IgG responses to the Aedes albopictus 34k2 salivary protein: Analyses in Reunion Island and Bolivia confirm its suitability as marker of host exposure to the tiger mosquito. Parasit. Vectors 15, 260. https://doi.org/10.1186/s13071-022-05383-8 (2022).
Li, Z. et al. Aedes albopictus salivary proteins adenosine deaminase and 34k2 interact with human mast cell specific proteases tryptase and chymase. Bioengineered 13, 13752–13766. https://doi.org/10.1080/21655979.2022.2081652 (2022).
Cox, J., Mota, J., Sukupolvi-Petty, S., Diamond, M. S. & Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 86, 7637–7649. https://doi.org/10.1128/JVI.00534-12 (2012).
McCracken, M. K., Christofferson, R. C., Chisenhall, D. M. & Mores, C. N. Analysis of early dengue virus infection in mice as modulated by Aedes aegypti probing. J. Virol. 88, 1881–1889. https://doi.org/10.1128/JVI.01218-13 (2014).
Schmid, M. A. et al. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody-dependent enhancement. PLoS Pathog. 12, e1005676. https://doi.org/10.1371/journal.ppat.1005676 (2016).
Sri-In, C. et al. A salivary protein of Aedes aegypti promotes dengue-2 virus replication and transmission. Insect Biochem. Mol. Biol. 111, 103181. https://doi.org/10.1016/j.ibmb.2019.103181 (2019).
Sun, P. et al. A mosquito salivary protein promotes flavivirus transmission by activation of autophagy. Nat. Commun. 11, 260. https://doi.org/10.1038/s41467-019-14115-z (2020).
Conway, M. J. et al. Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLoS Negl. Trop. Dis. 10, e0004941. https://doi.org/10.1371/journal.pntd.0004941 (2016).
Wei, Y. et al. Vector competence for DENV-2 among Aedes albopictus (Diptera: Culicidae) populations in China. Front. Cell. Infect. Microbiol. https://doi.org/10.3389/fcimb.2021.649975 (2021).
Luo, L. et al. The dengue preface to endemic in mainland China: The historical largest outbreak by Aedes albopictus in Guangzhou, 2014. Infect. Dis. Poverty 6, 148. https://doi.org/10.1186/s40249-017-0352-9 (2017).
Su, J. et al. Screening for differentially expressed miRNAs in Aedes albopictus (Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells. Parasit. Vectors 12, 44. https://doi.org/10.1186/s13071-018-3261-2 (2019).
Dolezelova, E., Zurovec, M., Dolezal, T., Simek, P. & Bryant, P. J. The emerging role of adenosine deaminases in insects. Insect Biochem. Mol. Biol. 35, 381–389. https://doi.org/10.1016/j.ibmb.2004.12.009 (2005).
Gao, Z.-W. et al. The roles of adenosine deaminase in autoimmune diseases. Autoimmunity Rev. https://doi.org/10.1016/j.autrev.2020.102709 (2021).
Flinn, A. M. & Gennery, A. R. Adenosine deaminase deficiency: A review. Orphanet. J. Rare Dis. 13, 65. https://doi.org/10.1186/s13023-018-0807-5 (2018).
Ribeiro, J. M., Charlab, R. & Valenzuela, J. G. The salivary adenosine deaminase activity of the mosquitoes Culex quinquefasciatus and Aedes aegypti. J. Exp. Biol. 204, 2001–2010. https://doi.org/10.1242/jeb.204.11.2001 (2001).
Ribeiro, J. M. & Valenzuela, J. G. The salivary purine nucleosidase of the mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 33, 13–22. https://doi.org/10.1016/s0965-1748(02)00078-4( (2003).
Surasombatpattana, P. et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J. Investig. Dermatol. 134, 281–284. https://doi.org/10.1038/jid.2013.251 (2014).
Chowdhury, A., Modahl, C. M., Misse, D., Kini, R. M. & Pompon, J. High resolution proteomics of Aedes aegypti salivary glands infected with either dengue, Zika or chikungunya viruses identify new virus specific and broad antiviral factors. Sci. Rep. 11, 23696. https://doi.org/10.1038/s41598-021-03211-0 (2021).
Arca, B. et al. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem. Mol. Biol. 37, 107–127. https://doi.org/10.1016/j.ibmb.2006.10.007 (2007).
Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651. https://doi.org/10.1101/cshperspect.a001651 (2009).
Chen, L. et al. BAY 11–7082, a nuclear factor-kappaB inhibitor, induces apoptosis and S phase arrest in gastric cancer cells. J. Gastroenterol. 49, 864–874. https://doi.org/10.1007/s00535-013-0848-4 (2014).
Totzke, J. et al. Takinib, a selective tak1 inhibitor, broadens the therapeutic efficacy of TNF-alpha inhibition for cancer and autoimmune disease. Cell Chem. Biol. 24, 1029-1039.e1027. https://doi.org/10.1016/j.chembiol.2017.07.011 (2017).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 11, 373–384. https://doi.org/10.1038/ni.1863 (2010).
Benelli, G., Wilke, A. B. B. & Beier, J. C. Aedes albopictus (Asian tiger mosquito). Trends Parasitol. 36, 942–943. https://doi.org/10.1016/j.pt.2020.01.001 (2020).
Machain-Williams, C. et al. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol. 34, 15–22. https://doi.org/10.1111/j.1365-3024.2011.01339.x (2012).
Pingen, M., Schmid, M. A., Harris, E. & McKimmie, C. S. Mosquito biting modulates skin response to virus infection. Trends Parasitol. 33, 645–657. https://doi.org/10.1016/j.pt.2017.04.003 (2017).
Bagheri, S., Saboury, A. A. & Haertlé, T. Adenosine deaminase inhibition. Int. J. Biol. Macromol. 141, 1246–1257. https://doi.org/10.1016/j.ijbiomac.2019.09.078 (2019).
Li, S. & Aksoy, S. A family of genes with growth factor and adenosine deaminase similarity are preferentially expressed in the salivary glands of Glossina m. morsitans. Gene 252, 83–93. https://doi.org/10.1016/s0378-1119(00)00226-2 (2000).
Charlab, R., Rowton, E. D. & Ribeiro, J. M. The salivary adenosine deaminase from the sand fly Lutzomyia longipalpis. Exp. Parasitol. 95, 45–53. https://doi.org/10.1006/expr.2000.4503 (2000).
Valenzuela, J. G., Pham, V. M., Garfield, M. K., Francischetti, I. M. B. & Ribeiro, J. M. C. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 32, 1101–1122. https://doi.org/10.1016/s0965-1748(02)00047-4 (2002).
Hasko, G. et al. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 164, 1013–1019. https://doi.org/10.4049/jimmunol.164.2.1013 (2000).
Guerrero, D., Cantaert, T. & Misse, D. Aedes mosquito salivary components and their effect on the immune response to arboviruses. Front. Cell Infect. Microbiol. 10, 407. https://doi.org/10.3389/fcimb.2020.00407 (2020).
Spencer Clinton, J. L. et al. Sialokinin in mosquito saliva shifts human immune responses towards intracellular pathogens. PLoS Negl. Trop. Dis. 17, e0011095. https://doi.org/10.1371/journal.pntd.0011095 (2023).
Costa, D. J. et al. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infect. Immun. 72, 1298–1305. https://doi.org/10.1128/IAI.72.3.1298-1305.2004 (2004).
Deshmane, S. L., Kremlev, S., Amini, S. & Sawaya, B. E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 29, 313–326. https://doi.org/10.1089/jir.2008.0027 (2009).
Hastings, A. K. et al. Aedes aegypti NeSt1 protein enhances zika virus pathogenesis by activating neutrophils. J. Virol. https://doi.org/10.1128/JVI.00395-19 (2019).
Besson, B. et al. Arbovirus-vector protein interactomics identifies Loquacious as a co-factor for dengue virus replication in Aedes mosquitoes. PLoS Pathog. 18, e1010329. https://doi.org/10.1371/journal.ppat.1010329 (2022).
Caraballo, G. I. et al. The dengue virus nonstructural protein 1 (NS1) interacts with the putative epigenetic regulator DIDO1 to promote flavivirus replication in mosquito cells. J. Virol. 96, e0070422. https://doi.org/10.1128/jvi.00704-22 (2022).
Rubio-Miranda, J. A. et al. Septin 2 interacts with dengue virus replication complex proteins and participates in virus replication in mosquito cells. Virology 570, 67–80. https://doi.org/10.1016/j.virol.2022.03.007 (2022).
Fernandes-Santos, C. & Azeredo, E. L. Innate immune response to dengue virus: Toll-like receptors and antiviral response. Viruses https://doi.org/10.3390/v14050992 (2022).
Thompson, M. R., Kaminski, J. J., Kurt-Jones, E. A. & Fitzgerald, K. A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 3, 920–940. https://doi.org/10.3390/v3060920 (2011).
Cheng, J. et al. Cloning and characterization of a mannose binding C-type lectin gene from salivary gland of Aedes albopictus. Parasit. Vectors 7, 337. https://doi.org/10.1186/1756-3305-7-337 (2014).
Acknowledgements
We thank Dr. Tongyan Zhao for her kindness in providing the Guangzhou strain of Ae. albopictus Aedes albopictus.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81971972) and Guizhou Science and Technology Agency; (ZK [2021]430).
Author information
Authors and Affiliations
Contributions
J.H.W. and Z.Q.L. conceived and designed the project. Y.S. prepared and developed the 2DE gels and analyzed the profiles using dedicated software. L.C., Q.Q.L.V., and X.Y.K. performed the enzyme assays experiments. C.J.J. prepared Soluble recombinant protein ADA. L.Z.M. performed ELISA and virus preparation. J.Z.C., Y.N., W.Y.L. analyzed the data. M.X.H. performed western blotting and quantitative Real-time PCR experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mu, X., Lin, Z., Sun, Y. et al. Aedes albopictus salivary adenosine deaminase is an immunomodulatory factor facilitating dengue virus replication. Sci Rep 13, 16660 (2023). https://doi.org/10.1038/s41598-023-43751-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43751-1
- Springer Nature Limited