Abstract
Background
The rapid worldwide spreading of Aedes aegypti and Aedes albopictus is expanding the risk of arboviral diseases transmission, pointing out the urgent need to improve monitoring and control of mosquito vector populations. Assessment of human-vector contact, currently estimated by classical entomological methods, is crucial to guide planning and implementation of control measures and evaluate transmission risk. Antibody responses to mosquito genus-specific salivary proteins are emerging as a convenient complementary tool for assessing host exposure to vectors. We previously showed that IgG responses to the Ae. albopictus 34k2 salivary protein (al34k2) allow detection of seasonal and geographic variation of human exposure to the tiger mosquito in two temperate areas of Northeast Italy. The main aim of this study was to confirm and extend these promising findings to tropical areas with ongoing arboviral transmission.
Methods
IgG responses to al34k2 and to the Ae. aegypti orthologous protein ae34k2 were measured by ELISA in cohorts of subjects only exposed to Ae. albopictus (Réunion Island), only exposed to Ae. aegypti (Bolivia) or unexposed to both these vectors (North of France).
Results and conclusion
Anti-al34k2 IgG levels were significantly higher in sera of individuals from Réunion Island than in unexposed controls, indicating that al34k2 may be a convenient and reliable proxy for whole saliva or salivary gland extracts as an indicator of human exposure to Ae. albopictus. Bolivian subjects, exposed to bites of Ae. aegypti, carried in their sera IgG recognizing the Ae. albopictus al34k2 protein, suggesting that this salivary antigen can also detect, even though with low sensitivity, human exposure to Ae. aegypti. On the contrary, due to the high background observed in unexposed controls, the recombinant ae34k2 appeared not suitable for the evaluation of human exposure to Aedes mosquitoes. Overall, this study confirmed the suitability of anti-al34k2 IgG responses as a specific biomarker of human exposure to Ae. albopictus and, to a certain extent, to Ae. aegypti. Immunoassays based on al34k2 are expected to be especially effective in areas where Ae. albopictus is the main arboviral vector but may also be useful in areas where Ae. albopictus and Ae. aegypti coexist.
Graphical Abstract

Similar content being viewed by others
Background
Arboviral diseases transmitted by mosquitoes (such as dengue, chikungunya, Zika, West Nile or yellow fever) are a serious concern for public health, and > 50% of the world’s population is considered at risk of contracting at least one of these arbovirosis [1]. Except for West Nile virus, commonly transmitted by Culex mosquitoes, all other arboviruses mentioned above are transferred to humans by Aedes species. The major vector is certainly the yellow fever mosquito Aedes aegypti, but the Asian tiger mosquito Aedes albopictus can play a relevant vectorial role, especially in areas where Ae. aegypti is absent or present at low density [2,3,4]. The geographical distribution of these two species increased impressively during the past decades [5], extending the risk of Aedes-borne arboviral diseases to temperate areas [6], as well illustrated by the repeated cases of chikungunya (CHIKV), dengue (DENV) and Zika virus (ZIKAV) transmission in continental Europe [7,8,9]. No specific anti-viral drugs are currently available against these arboviral diseases. A safe and effective vaccine against yellow fever has been available since the end of 1930s, and a dengue vaccine was recently licensed; nevertheless, yellow fever is still endemic in several tropical countries [10], and the use of the dengue vaccine has relevant limitations [11, 12]. For these reasons, the containment of these arboviral diseases relies almost exclusively on vector monitoring and control and on reducing the contact between the human host and the mosquito vector. Insecticides are still the most effective weapon for vector control. However, the spreading of resistance among mosquito vector populations points to the need for a careful management of insecticides [13] and for the development of novel approaches [14, 15]. In this respect, very promising results have been obtained by Wolbachia-infected Ae. aegypti for the containment of dengue [16, 17].
The evaluation of human-vector contact is of fundamental importance to monitor Aedes populations, to assess the risk of arboviral transmission and for planning/implementation of vector control measures. This is usually carried out by classical entomological methods [18], which, however, have some intrinsic limitations since they can be labor-/time-consuming, may raise ethical concerns (human landing catches) and only provide indirect estimations at the community level. The use of IgG responses to mosquito salivary proteins emerged as a convenient complementary or alternative tool to evaluate human-vector contact and assess the risk of mosquito-borne diseases. First demonstrations used mosquito saliva or salivary gland extracts (SGE) [19,20,21,22]; later on, the identification of Anopheles- and Aedes-specific salivary proteins [23, 24] paved the way for the development of immunoassays based on single genus-specific salivary proteins, with improvement of reproducibility and minimization of potential cross-reactivities. IgG responses to the Anopheles gambiae salivary protein gSG6, or the derived gSG6-P1 peptide, were shown to be reliable indicators of human exposure to malaria vectors in several different epidemiological settings [25,26,27,28,29,30,31,32], and a few additional anopheline salivary proteins have been tested or proposed as candidate markers [33,34,35]. These encouraging results stimulated the development of similar tools for Aedes vectors [36, 37], and promising indications have been obtained with the Nterm-34 kDa peptide, which is designed on the culicine-specific 34k1 salivary protein from Ae. aegypti and could detect human exposure to Ae. aegypti and possibly to Ae. albopictus [38,39,40,41,42].
We focused our attention on another member of the culicine-specific 34-kDa family, the 34k2 salivary proteins of Ae. albopictus and Ae. aegypti, which have limited amino acid identity to 34k1 (32–33%) and to orthologues from Culex species (< 30%) [43, 44]. Using a murine model, we initially showed that recombinant 34k2 proteins from Ae. albopictus (al34k2) and Ae. aegypti (ae34k2) were immunogenic to mice; intriguingly, sera of mice experimentally immunized to Ae. albopictus saliva did not carry IgG antibodies recognizing the ae34k2 protein, and vice versa, indicating the absence of immune cross-reactivity [45]. Moreover, using sera collected from adult healthy donors in two Ae. albopictus colonized areas of Northeast Italy (Padova and Belluno), we could show that anti-al34k2 IgG responses appeared appropriate to detect spatial and seasonal variation of human exposure to Ae. albopictus [46]. The aim of this study was to verify and validate, in a different epidemiological setting, the suitability of al34k2 for the assessment of human exposure to Ae. albopictus and to get additional insights into usefulness and suitability of 34k2 salivary proteins as universal or species-specific markers of human exposure to Aedes mosquitoes. To this end, we analyzed IgG responses to the al34k2 and ae34k2 salivary proteins in individuals from tropical areas with ongoing arboviral transmission. Specifically, in populations from Réunion Island, where individuals were naturally exposed only to Ae. albopictus, and from an area of Bolivia where only Ae. aegypti was present.
Methods
Ethics statement
All studies followed ethical principles as stipulated in the Edinburgh revision of the Helsinki Declaration. The studies in Réunion Island and the North of France were approved by a French Ethics Committee (the Sud Ouest, Outre Mer Ethics Committee, 25/ 02/2009) and authorized by the French Drug Agency (AFFSAPS, Ministry of Health; 12/01/2009). The study in Bolivia was approved by the Bolivian Committee of Bioethics (September 2006) and the Institut de Recherche pour le Dévelopement (IRD) "Comité Consultatif de Déontologie et d’Ethique" (July 2006). Written informed consent was obtained from every subject.
Study population
Sera analyzed in this study represent subsets of samples collected from adult individuals in Réunion Island and in Bolivia and used in previous investigations to measure IgG responses to Ae. albopictus and Ae. aegypti SGEs, respectively [20, 21]. The Bolivian subset (n = 115; age range 18–78, mean age 35.3 years old) was selected, according to availability and age > 18 years old, from a large group of sera collected from April to May 2007 in an urban area of Santa Cruz de la Sierra [21]. In this area Ae. aegypti was responsible for several dengue outbreaks in the years preceding the survey, and individuals were exposed to Ae. aegypti (but no to Ae. albopictus). Sera from Réunion Island (n = 108; age range 18–30, mean age 24.0 years old) were collected in the city of Le Tampon during the seasonal peak of Ae. albopictus (May–June 2009). Aedes aegypti populations are present in specific locations on Réunion Island [47], but they are absent in the study area where Ae. albopictus is widely spread and was responsible for the large chikungunya and dengue outbreaks in 2005 and 2018, respectively [3, 4]. Sera collected from healthy adult donors from a region in North of France, free of both Ae. albopictus and Ae. aegypti, were used as unexposed control groups: note that the cohorts C (n = 20) used in this study and cohort C1 (n = 18), previously employed by Doucoure and collaborators to measure IgG responses to SGE in Réunion Island and Bolivia [20, 21], were composed of different individuals.
Evaluation of human IgG Ab levels
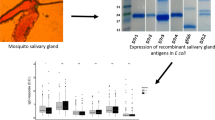
The recombinant 34k2 salivary proteins from Ae. albopictus (al34k2, AAV90690) and Ae. aegypti (ae34k2, AAL76018) were expressed in E. coli and purified by a double step of immobilized metal ion affinity chromatography (IMAC) followed by gel filtration as previously described [45]. Enzyme-linked immunosorbent assays (ELISA) were performed as previously described [45, 46] with very few modifications. Briefly, flat-bottom 96-well plates (Nunc MaxiSorp, 442404) were coated for 3 h at room temperature (RT) with 50 µl of recombinant protein (5 µg/ml) diluted in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 3 mM NaN3, pH 9.6). After washings, wells were incubated (3 h, RT) with blocking buffer [150 μl, 1% w/v skimmed dry milk in PBST (1 × PBS, 0.05% Tween 20)], washed again and then incubated overnight at 4 °C with 50 μl of serum diluted 1:25 in blocking buffer. After washings, plates were incubated (3 h, RT) with 100 μl of polyclonal rabbit anti-human IgG/horseradish peroxidase (HRP) antibody (Dako P0214) diluted 1:5000. After washing, the colorimetric development was carried out (15 min, 25 °C in the dark) with 100 μl of o-phenylenediamine dihydrochloride (OPD, Sigma P8287). The reaction was terminated adding 25 μl of 2 M H2SO, and the optical density at 492 nm (OD492) was determined using a Biotek Synergy HT microplate reader. Washings always consisted of a first washing with PBS-T followed by three additional washings with distilled water.
Normalization and data analysis
All samples were analyzed in duplicate with the antigen and once with no antigen for background subtraction. IgG levels, expressed as OD values at 492 nm, were calculated for each sample as the mean OD value with antigen minus the OD value without antigen. Samples with coefficient of variation between duplicates > 20% were retested or not included in the analysis. To control for intra- and inter-assay variation, each plate included negative controls and a standard curve made by three-fold dilution series (1:3–1:6561) of a pool of sera identified as hyperimmune to Ae. albopictus saliva in a previous study [46]. OD values were normalized using titration curves and the Excel software with a three variable sigmoid model and the Solver add-in application as previously described [48]. The mean OD values of unexposed controls plus 3xSD were used as cut-off values for seropositivity, and they were as follows: al34k2, 0.257; ae34k2, 1.017; alSGE, 0.269; aeSGE, 0.160. Prevalence was expressed as percentage ± 95% confidence interval. The Mann-Whitney U test and the Wilcoxon matched-pairs signed rank test were used for the pairwise comparisons among independent and paired groups, respectively. Proportions were compared by the Fisher’s exact test. Graph preparation and statistical analyses were performed using the Prism 8.0 GraphPad Software (San Diego, CA).
Results and discussion
IgG responses to al34k2 in individuals from Réunion Island naturally exposed to Aedes albopictus
Using sera collected in two areas colonized by Ae. albopictus in Northeast Italy, we previously found that IgG responses to the al34k2 salivary protein may be suitable as a marker to evaluate both geographical and temporal variation of human exposure to the tiger mosquito [46]. To reinforce these promising indications and provide a validation in an epidemiological setting with ongoing arboviral transmission, we determined IgG responses to al34k2 in a group of naturally exposed individuals from Réunion Island. This cohort appeared especially appropriate since IgG responses to Ae. albopictus salivary gland extracts (alSGE) had been previously measured in these same individuals by Doucoure and colleagues [20]. Moreover, in the area under study Ae. albopictus is the only Aedes species known to bite humans, whereas Ae. aegypti is absent. In these conditions IgG responses to al34k2 can be interpreted with minimal confounding effects due to exposure of the resident population to other Aedes species.
We found that IgG responses to al34k2 were significantly higher in exposed subjects from Réunion Island than in the unexposed control group (p < 0.0001, Fig. 1A), a result that is fully consistent with the difference in anti-alSGE IgG antibody levels previously measured in the same cohort of individuals by Doucoure and collaborators [20] (p < 0.0001, Fig. 1A). The remarkably higher anti-al34k2 IgG antibody levels in exposed subjects points to al34k2 as a highly valuable antigen to reveal human exposure to bites of the tiger mosquito Ae. albopictus, especially considering the characteristics of the study area and previous experimental evidence. First, sera were collected during the high mosquito density season in an area where Ae. albopictus is the only Aedes species known to bite humans, which facilitates result interpretation. Second, within the limits of a comparison between ELISA experiments performed independently and in different conditions, IgG antibody levels and seroprevalence to al34k2 (0.67 ± 0.089) were in good agreement with IgG responses and prevalence to alSGE (0.87 ± 0.063; Fig. 1B). The higher IgG responses and prevalence to alSGE, as well as the relatively low Spearman correlation coefficient between IgG responses to the two antigens (r = 0.50, 95% CI 0.34 to 0.63, p < 0.0001), should not be too surprising considering that (1) Aedes saliva is a complex mixture of 100–150 proteins [23] and (2) human immune response to mosquito salivary proteins is both quantitatively and qualitatively heterogeneous [49]. Notably, seroprevalence values to al34k2 (66.7%) and alSGE (78.0%) suggest that recombinant al34k2 can detect > 85% of individuals seropositive to alSGE, indicating that al34k2 may be a convenient and reliable proxy for whole saliva or SGE as an indicator of human exposure to the tiger mosquito.
IgG responses to al34k2 in individuals from Réunion Island and unexposed controls. A Scatter plot of IgG responses to al34k2 in subjects from Réunion Island (RE, n = 108) and in unexposed controls (C n = 20). IgG responses to alSGE in the same individuals (RE, n = 108) and in unexposed controls (C1 n = 18), as previously determined [20], are shown for visual comparison. Horizontal bars indicate median values. al34k2, Ae. albopictus 34k2 recombinant protein; alSGE, Ae. albopictus salivary gland extracts. IgG responses are expressed as OD values. The non-parametric Mann-Whitney test was used for the pairwise comparisons. ****p < 0.0001. B Seroprevalence of anti-al34k2 and anti-alSGE IgG antibodies. Error bars indicate 95% confidence intervals
IgG responses to al34k2 in subjects from Bolivia naturally exposed to Aedes aegypti
The 34k2 salivary proteins from Ae. albopictus and Ae. aegypti share a relatively high amino acid identity (62%), and understanding the degree of species-specificity of human anti-al34k2 IgG responses represents a question of interest. This is especially relevant considering that in a murine model the 34k2 proteins from Ae. albopictus (al34k2) and Ae. aegypti (ae34k2) were both immunogenic but, surprisingly, there was no cross-reactivity between these two antigens. In fact, sera of mice immunized to Ae. albopictus saliva did not include IgG antibodies recognizing the ae34k2 protein; vice versa, mice exposed to Ae. aegypti did not carry IgG antibodies able to recognize al34k2 [45]. The analyses of human cohorts from Northeast Italy [46] and from Réunion Island (see above), which refer to subjects naturally exposed to bites of Ae. albopictus, provided no clues on the potential usefulness of the al34k2 protein to reveal human exposure to Ae. aegypti. To gain some insights into this issue we analyzed IgG responses to al34k2 in a group of individuals from Bolivia who were exposed to bites of Ae. aegypti but not of Ae. albopictus. Notably, these samples represent a subset of sera from a larger study designed to evaluate specific IgG antibody responses to Ae. aegypti salivary gland extracts (aeSGE) [21]. Therefore, this Bolivian cohort appeared to be a perfect complement of the Réunion Island cohort described before, because of both the specificity of exposure to Ae. aegypti and the availability of data on IgG responses to aeSGE.
Interestingly, anti-al34k2 IgG levels were higher in sera of individuals from Bolivia than in the unexposed cohort (p = 0.0014, Fig. 2A). These same subjects, as previously determined by Doucoure and collaborators [21], showed significantly higher IgG responses to Ae. aegypti salivary gland extracts compared to unexposed controls (p < 0.0001). These observations suggest that individuals exposed to Ae. aegypti develop IgG antibodies against ae34k2 which can recognize, at least to a certain extent, the Ae. albopictus 34k2 protein. However, this cross-recognition appeared rather weak, as suggested by the low median anti-al34k2 IgG value (below the cut-off level) and by the low prevalence value (0.22 ± 0.038) compared to aeSGE (0.73 ± 0.042; Fig. 2B). Overall, these observations suggest that the al34k2 antigen from Ae. albopictus may be able to detect exposure to Ae. aegypti; however, sensitivity may be too low as indicated by the fact that recombinant al34k2 can only detect around 30% of individuals seropositive to aeSGE.
IgG responses to al34k2 in individuals from Bolivia and unexposed controls. A Scatter plot of IgG responses to al34k2 in subjects from Bolivia (BO, n = 115) and in unexposed controls (C, n = 20). IgG responses to aeSGE in the same individuals (BO, n = 115) and in unexposed controls (C1 n = 18), as previously determined (21), are shown for visual comparison. Horizontal bars indicate median values. al34k2, Ae. albopictus 34k2 recombinant protein; aeSGE, Ae. aegypti salivary gland extracts. IgG responses are expressed as OD values. The non-parametric Mann-Whitney test was used for the pairwise comparisons. **p < 0.01; ****p < 0.0001. B Seroprevalence of anti-al34k2 and anti-aeSGE IgG antibodies. Error bars indicate 95% confidence intervals
IgG responses to ae34k2 in subjects from Bolivia, Réunion Island and in unexposed controls
The two cohorts from Réunion Island and Bolivia, due to their peculiarities, also represented an ideal sample to evaluate IgG responses to the Ae. aegypti 34k2 salivary protein, which we had previously shown to be immunogenic to mice [45]. Surprisingly, anti-ae34k2 IgG levels were significantly higher than anti-al34k2 IgG levels in sera of unexposed individuals (p = 0.0003, Wilcoxon matched-pair test) but not significantly different between unexposed and exposed, from either Réunion Island or Bolivia (p > 0.05, Mann–Whitney test; Fig. 3). The different response of the unexposed control group to al34k2 (mean 0.037, median 0.000) and ae34k2 (mean 0.252, median 0.153) suggests a high background level with the ae34k2 antigen, something that we did not observe in previous experiments in mice [45]. We do not know the reason for this high background observed with human sera, possibly due to cross-reactivity to some unknown antigen(s). However, note that the two recombinant proteins, which were expressed in E. coli and purified with essentially identical experimental procedures [45], had only minimal and similar traces of endotoxin, as quantified by the Limulus Amebocyte Lysate assay (Pierce 88,282). According to these observations, the use of the Ae. aegypti ae34k2 salivary protein, or at least of this recombinant version, does not appear suitable for the development of immunoassays to evaluate human exposure to Aedes mosquitoes.
IgG responses to ae34k2 in individuals from Réunion Island, Bolivia and in unexposed controls. Scatter plot of IgG responses to ae34k2 in unexposed controls (C, n = 20), in subjects from Réunion Island (RE, n = 108) and from Bolivia (BO, n = 115). IgG responses to al34k2 of unexposed controls (C, n = 20) are shown for comparison. Horizontal bars indicate median values. al34k2, Ae. albopictus 34k2 recombinant protein; ae34k2, Ae. aegypti 34k2 recombinant protein. IgG responses are expressed as OD values. The non-parametric Wilcoxon matched pair signed rank test was used for the pairwise comparisons in unexposed controls. ***p < 0.001
Conclusions
In conclusion, this study consolidates previous observations made in temperate areas of Northeast Italy [46] and clearly establishes the reliability and specificity of anti-al34k2 IgG responses to evaluate human exposure to Ae. albopictus. Importantly, the results obtained in Réunion Island extend the possible use of the al34k2 antigen to tropical and subtropical areas, where dengue or chikungunya transmissions are endemic and arboviral circulation is maintained by the tiger mosquito. This opens new scenarios for the development of protocols that may complement classical entomological measures. In fact, the serological evaluation of human exposure to Ae. albopictus may allow to improve monitoring and control of vector populations and possibly provide novel tools for arboviral transmission risk assessment. In particular, IgG responses to al34k2 may help to estimate efficacy of interventions against the tiger mosquito, from classical insecticide-based control of larval and adult stages to more environmentally friendly alternatives as the sterile insect technique (SIT), which is actually under implementation in Réunion Island [50]. The ability of the al34k2 antigen to reveal, at least in part, also exposure to Ae. aegypti suggests its possible use even in areas where both vectors are present. However, considering the low level of cross-reactivity, a possible perspective may be the development of immunoassays based on a combination of the Nterm-34 kDa peptide, which is designed on ae34k2, and of the al34k2 protein. This may provide an assay capable to reveal with higher sensitivity human exposure to both Ae. aegypti and Ae. albopictus. Overall, the addition to our portfolio of novel mosquito salivary antigens suitable for the evaluation of human-vector contact is expected to significantly enrich our toolbox for the control of mosquito-borne diseases. Such complementary tools, which can be employed for epidemiological studies, and possibly for the evaluation of transmission risk, may be especially useful when implementation of classical entomological methods is challenging (low vector density, logistic constraints, limited resources, etc.) or when the simultaneous determination of exposure to vector and to specific circulating pathogen(s) by serological measurements may be needed.
Availability of data and materials
Data generated during this study are included in this published article (and its supplementary information files). The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
WHO. Global vector control response 2017–2030. Geneva: World Health Organization 2017. Available from: http://apps.who.int/iris/bitstream/10665/259205/1/9789241512978-eng.pdf.
Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10:259–66.
Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–31.
Vincent M, Larrieu S, Vilain P, Etienne A, Solet JL, Francois C, et al. From the threat to the large outbreak: dengue on reunion Island, 2015 to 2018. Euro Surveill. 2019. https://doi.org/10.2807/1560-7917.ES.2019.24.47.1900346.
Kraemer MUG, Reiner RC Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63.
Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–55.
Brady OJ, Hay SI. The first local cases of Zika virus in Europe. Lancet. 2019;394:1991–2.
Gossner CM, Ducheyne E, Schaffner F. Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. Euro Surveill. 2018;23:1800268.
Lazzarini L, Barzon L, Foglia F, Manfrin V, Pacenti M, Pavan G, et al. First autochthonous dengue outbreak in Italy, August 2020. Euro Surveill. 2020;25(36).
WHO. World Health Organization. yellow fever fact sheets. 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/yellow-fever.
CDC. Center for disease control and prevention. dengue vaccine. 2019. Available from: https://www.cdc.gov/dengue/prevention/dengue-vaccine.html.
Espana G, Yao Y, Anderson KB, Fitzpatrick MC, Smith DL, Morrison AC, et al. Model-based assessment of public health impact and cost-effectiveness of dengue vaccination following screening for prior exposure. PLoS Negl Trop Dis. 2019;13:e0007482.
Dusfour I, Vontas J, David JP, Weetman D, Fonseca DM, Corbel V, et al. Management of insecticide resistance in the major aedes vectors of arboviruses: advances and challenges. PLoS Negl Trop Dis. 2019;13:e0007615.
Oliva CF, Benedict MQ, Collins CM, Baldet T, Bellini R, Bossin H, et al. Sterile insect technique (SIT) against Aedes Species mosquitoes: a roadmap and good practice framework for designing, implementing and evaluating pilot field trials. Insects. 2021;12:191.
Shaw WR, Catteruccia F. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat Microbiol. 2019;4:20–34.
Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wmel wolbachia in aedes aegypti mosquitoes and reduction of local dengue transmission in cairns and surrounding locations in northern queensland. Australia Gates Open Res. 2019;3:1547.
Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. 2021;384:2177–86.
ECDC. European centre for disease prevention and control. guidelines for the surveillance of invasive mosquitoes in Europe.2012. Available from: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf.
Andrade BB, Rocha BC, Reis-Filho A, Camargo LM, Tadei WP, Moreira LA, et al. Anti-Anopheles darlingi saliva antibodies as marker of Plasmodium vivax infection and clinical immunity in the Brazilian Amazon. Malar J. 2009;8:121.
Doucoure S, Mouchet F, Cornelie S, DeHecq JS, Rutee AH, Roca Y, et al. Evaluation of the human IgG antibody response to Aedes albopictus saliva as a new specific biomarker of exposure to vector bites. PLoS Negl Trop Dis. 2012;6:e1487.
Doucoure S, Mouchet F, Cournil A, Le Goff G, Cornelie S, Roca Y, et al. Human antibody response to Aedes aegypti saliva in an urban population in bolivia: a new biomarker of exposure to dengue vector bites. Am J Trop Med Hyg. 2012;87:504–10.
Remoue F, Cisse B, Ba F, Sokhna C, Herve JP, Boulanger D, et al. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg. 2006;100:363–70.
Arcà B, Ribeiro JM. Saliva of hematophagous insects: a multifaceted toolkit. Curr Opin Insect Sci. 2018;29:102–9.
Ribeiro JM, Mans BJ, Arcà B. An insight into the sialome of blood-feeding Nematocera. Insect Biochem Mol Biol. 2010;40:767–84.
Badu K, Siangla J, Larbi J, Lawson BW, Afrane Y, Ong’echa J, et al. Variation in exposure to Anopheles gambiae salivary gland peptide (gSG6-P1) across different malaria transmission settings in the western Kenya highlands. Malar J. 2012;11:318.
Idris ZM, Chan CW, Mohammed M, Kalkoa M, Taleo G, Junker K, et al. Serological measures to assess the efficacy of malaria control programme on Ambae Island, Vanuatu. Parasit Vectors. 2017;10:204.
Montiel J, Carbal LF, Tobon-Castano A, Vasquez GM, Fisher ML, Londono-Renteria B. IgG antibody response against anopheles salivary gland proteins in asymptomatic plasmodium infections in Narino, Colombia. Malar J. 2020;19:42.
Poinsignon A, Cornelie S, Mestres-Simon M, Lanfrancotti A, Rossignol M, Boulanger D, et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to anopheles bites. PLoS ONE. 2008;3:e2472.
Pollard EJM, Patterson C, Russell TL, Apairamo A, Oscar J, Arca B, et al. Human exposure to anopheles farauti bites in the solomon islands is not associated with igg antibody response to the gsg6 salivary protein of anopheles gambiae. Malar J. 2019;18:334.
Rizzo C, Ronca R, Fiorentino G, Verra F, Mangano V, Poinsignon A, et al. Humoral response to the Anopheles gambiae salivary protein gSG6: a serological indicator of exposure to afrotropical malaria vectors. PLoS ONE. 2011;6:e17980.
Stone W, Bousema T, Jones S, Gesase S, Hashim R, Gosling R, et al. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS ONE. 2012;7:e40170.
Ya-Umphan P, Cerqueira D, Parker DM, Cottrell G, Poinsignon A, Remoue F, et al. Use of an anopheles salivary biomarker to assess malaria transmission risk along the thailand-myanmar border. J Infect Dis. 2017;215:396–404.
Ali ZM, Bakli M, Fontaine A, Bakkali N, Vu Hai V, Audebert S, et al. Assessment of anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar J. 2012;11:439.
Arcà B, Lombardo F, Struchiner CJ, Ribeiro JM. Anopheline salivary protein genes and gene families: an evolutionary overview after the whole genome sequence of sixteen Anopheles species. BMC Genomics. 2017;18:153.
Londono-Renteria B, Drame PM, Montiel J, Vasquez AM, Tobon-Castano A, Taylor M, et al. Identification and pilot evaluation of salivary peptides from anopheles albimanus as biomarkers for bite exposure and malaria infection in Colombia. Int J Mol Sci. 2020;21:691.
Londono-Renteria BL, Shakeri H, Rozo-Lopez P, Conway MJ, Duggan N, Jaberi-Douraki M, et al. Serosurvey of human antibodies recognizing Aedes aegypti D7 salivary proteins in Colombia. Front Public Health. 2018;6:111.
Sagna AB, Yobo MC, Elanga Ndille E, Remoue F. New immuno-epidemiological biomarker of human exposure to aedes vector bites: from concept to applications. Trop Med Infect Dis. 2018;3:80.
Elanga Ndille E, Doucoure S, Damien G, Mouchet F, Drame PM, Cornelie S, et al. First attempt to validate human IgG antibody response to Nterm-34kDa salivary peptide as biomarker for evaluating exposure to Aedes aegypti bites. PLoS Negl Trop Dis. 2012;6:e1905.
Elanga Ndille E, Doucoure S, Poinsignon A, Mouchet F, Cornelie S, D’Ortenzio E, et al. Human IgG antibody response to aedes nterm-34kDa salivary peptide, an epidemiological tool to assess vector control in chikungunya and dengue transmission area. PLoS Negl Trop Dis. 2016;10:e0005109.
Fustec B, Phanitchat T, Aromseree S, Pientong C, Thaewnongiew K, Ekalaksananan T, et al. Serological biomarker for assessing human exposure to aedes mosquito bites during a randomized vector control intervention trial in northeastern thailand. PLoS Negl Trop Dis. 2021;15:e0009440.
Ndille EE, Dubot-Peres A, Doucoure S, Mouchet F, Cornelie S, Sidavong B, et al. Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: a retrospective study in urban settings of Vientiane city. Lao PDR Trop Med Int Health. 2014;19:576–80.
Sagna AB, Kassie D, Couvray A, Adja AM, Hermann E, Riveau G, et al. Spatial assessment of contact between humans and anopheles and aedes mosquitoes in a medium-sized african urban setting, using salivary antibody-based biomarkers. J Infect Dis. 2019;220:1199–208.
Arcà B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, et al. An insight into the sialome of the adult female mosquito aedes albopictus. Insect Biochem Mol Biol. 2007;37:107–27.
Ribeiro JM, Arcà B, Lombardo F, Calvo E, Phan VM, Chandra PK, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6.
Buezo Montero S, Gabrieli P, Severini F, Picci L, Di Luca M, Forneris F, et al. Analysis in a murine model points to IgG responses against the 34k2 salivary proteins from aedes albopictus and Aedes aegypti as novel promising candidate markers of host exposure to aedes mosquitoes. PLoS Negl Trop Dis. 2019;13:e0007806.
Buezo Montero S, Gabrieli P, Montarsi F, Borean A, Capelli S, De Silvestro G, et al. IgG antibody responses to the aedes albopictus 34k2 salivary protein as novel candidate marker of human exposure to the tiger mosquito. Front Cell Infect Microbiol. 2020;10:377.
Bagny Beilhe L, Delatte H, Juliano SA, Fontenille D, Quilici S. Ecological interactions in Aedes species on reunion Island. Med Vet Entomol. 2013;27:387–97.
Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195.
Rizzo C, Lombardo F, Ronca R, Mangano V, Sirima SB, Nebie I, et al. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors. 2014;7:549.
Gouagna LC, Damiens D, Oliva CF, Boyer S, Le Goff G, Brengues C, et al. Strategic approach advances, and challenges in the development and application of the sit for area-wide control of Aedes albopictus mosquitoes in reunion island. Insects. 2020;11:770.
Acknowledgements
The authors gratefully acknowledge the population of Réunion, Santa Cruz de la Sierra and the French volunteers for their participation in this study. A special thanks to Dr. Françoise Mathieu-Daude for the generous help and the useful discussions and to Prof. Federico Forneris for the precious contribution with protein expression and purification.
Funding
This work was partly funded by funds from the Italian Ministry of Defense (PNRM 2017-SENSOR) to Marco Pombi and BA. SBM was supported by a fellowship from the PhD program in Infectious Diseases, Microbiology and Public Health (Sapienza University of Rome), and BZHZ by a PhD fellowship provided by the Méditerannée Infection Foundation (France). PG was supported from the Fondazione Cariplo (Project 2017–0798).
Author information
Authors and Affiliations
Contributions
BA conceived the study. AP, FR, FL, and BA designed the experiments. SBM and BZHZ performed the experiments. PG provided the al34k2 and ae34k2 antigens. AP and FR provided the sera. SBM, AP, FL and BA analyzed the data. SBM and BA performed the statistical analysis. BA wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the final submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All studies followed the ethical principles of the Helsinki Declaration and were approved by the competent Ethical Committees as specified in the Methods section.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Buezo Montero, S., Gabrieli, P., Poinsignon, A. et al. Human IgG responses to the Aedes albopictus 34k2 salivary protein: analyses in Réunion Island and Bolivia confirm its suitability as marker of host exposure to the tiger mosquito. Parasites Vectors 15, 260 (2022). https://doi.org/10.1186/s13071-022-05383-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05383-8