Abstract
Cadmium is a highly neurotoxic heavy metal that disrupts membranes and causes oxidative stress in the brain. The study aimed to investigate the neuroprotective effect of gallic acid on oxidative damage in the brains of Wistar rats exposed to cadmium chloride (CdCl2). Male Wistar rats were divided into four groups of five rats each. Group 1 was administered distilled water only throughout the study. Throughout the study, Group 2 received CdCl2 alone (5 mg/kg b.w./day), Group 3 received gallic acid (20 mg/kg b.w./day), and Group 4 received CdCl2 + gallic acid (20 mg/kg). Treatments were oral with distilled water as a vehicle. The study lasted 21 days. In the brain, the activities of cholinesterase and antioxidant enzymes were evaluated, as well as the levels of reduced glutathione, malondialdehyde, neurotransmitters, Na+/K+ ATPase, myeloperoxidase activity, nitric oxide, and interleukin-6. CdCl2-induced brain impairments in experimental animals and gallic acid prevents the following CdCl2-induced activities: inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), elevated neurotransmitters (serotonin and dopamine), decreased antioxidant enzymes (superoxide dismutase, catalase), decreased glutathione, Na+/K+ ATPases, and increased MDA and neuroinflammatory markers (myeloperoxidase (MPO), nitric oxide, and interleukin-6 in the brain of experimental rats exposed to CdCl2 (p < 0.05). Taken together, the neuroprotective effects of gallic acid on CdCl2-induced toxicity in the brains of rats suggest its potent antioxidant and neurotherapeutic properties.
Similar content being viewed by others
Introduction
Cadmium (Cd) is a toxic heavy metal that has a negative impact on human and animal cellular and metabolic systems. Cd levels in brain tissue are always high in rats that have been intoxicated with the metal (at dosage roughly 700 times higher than the control values). Cadmium salts is one of the most toxic pollutants found in the environment. Because Cd is not broken down, the danger of human exposure to Cd via food chain pollution is constantly increasing1. Exposure to cadmium occurs primarily through foods such as cereals and vegetables, implying that exposure is constant. The rise in Cd contamination is a major public health concern around the world2. Long-term exposure to Cd is well known to cause toxic side effects in numerous organ systems, like the cardiovascular, brain, and immune/hemopoietic3,4. Cd has the potential to cause neurotoxicity, resulting in an extensive range of clinical entities such as neurological disturbances and changes in normal brain neurochemistry5. Cd has been shown to promote lipid peroxidation (LPO) by increasing free radicals6. Cd toxicity has been linked to the production of reactive oxygen species (ROS) and the depletion of antiradicals7. The capability of Cd to cause oxidative stress in brain cells has been described as ROS generation after Cd intoxication with mitochondrial sites, resulting in the breakdown of mitochondrial potential and a subsequent decrease in intracellular glutathione levels8. Due to its high rate of oxygen utilization, abundance of polyunsaturated fatty acids, weak antioxidant defense, and high concentration of transition metals like copper and iron in some areas, brain tissue is highly susceptible to lipid peroxidation9. Loss of membrane-bound ATPase activity may occur as a result of the brain membranes' increased sensitivity to LPO10. Lipid-dependent membrane-bound enzymes known as ATPases play a role in active transport, preservation of cell homeostasis, and neurotransmission11. Cadmium modifies the activities of oxidative stress-neutralizing enzymes, causing disruptions in brain metabolism and contributing to cadmium’s neurotoxic effect. Cadmium causes changes in the structural integrity of lipids and has indirect effects on membrane-bound enzymes by increasing the production of free radicals in the brain and interfering with the antioxidant defense system12. The ability of Cd to cross the blood–brain barrier may explain its accumulation in brain tissue13. Reactive oxygen species (ROS) production is a normal process in metabolism. Excessive ROS production, not compensated by antioxidants, cause oxidative stress14. Presently, a broad investigation is being conducted to assess the preventive effects of many natural antioxidants on metal-induced toxicities15,16. Antioxidants are gaining increasing attention in the treatment of diseases related to oxidative stress as well as being probable therapeutic agents for a variety of disorders. Antioxidants can mitigate the cadmium-induced reduction in ATPase activity and the elevation of oxidative injury17.
One of the well-studied antioxidant agents is gallic acid. Gallic acid is a common plant metabolite that has the structure of trihydroxybenzoic acid and several hydrogen atoms in its phenolic structure that easily delocalize free radicals18,19. Its structure explains why it has strong antioxidant properties, indicating that it can protect tissues and organs from oxidative stress18,20. Gallic acid has previously been reported to be a common component of various foods and herbal drugs21. GA protects the brain by increasing antioxidant enzymes and decreasing inflammation22,23. Gallic acid can reduce the incidence of inflammation-related diseases, including cancer24,25, cardiovascular disease26,27, liver disease28,29, inflammation30,31, and neurodegenerative diseases. Gallic acid can inhibit the inflammatory process by eliminating superoxide anions, inhibiting the release and activity of myeloperoxidase (MPO), and possibly participating in the accumulation of active NADPH-oxidase31. This suggests that gallic acid is a promising chemical agent with a variety of chemotherapeutic properties. Given gallic acid’s antioxidant and anti-inflammatory properties, it is worth considering gallic acid as a potential preventive agent against cadmium-induced oxidative stress in the brain. The purpose of the study was to examine the protective role of gallic acid against oxidative damage caused by CdCl2 in the brains of male Wistar rats. As a result, we investigated the effects of gallic acid on the activities of AChE, BChE, and Na+/K+ ATPase. Furthermore, we examine the protective abilities of gallic acid on antioxidant enzymes (catalase and SOD) as well as GSH levels, markers of oxidative stress (TBA Reactive Substances (TBARS)), neurotransmitters (serotonin and dopamine levels), and inflammatory markers (MPO, nitric oxide (NO), and IL-6) in the brains of rats treated with CdCl2 to give more scientific evidence on the protective role of gallic acid.
Results
Gallic acid enhanced antioxidant status in cadmium chloride-induced Wistar rats
Figure 1 shows the effects of a 21-day exposure to CdCl2 and gallic acid on SOD, catalase, and GSH in the cerebrum of Wistar rats. CdCl2 alone was found to significantly (p < 0.05) reduce SOD, catalase, and GSH levels. On the other hand, CdCl2, markedly (p < 0.05) raised MDA levels in the cerebrum of experimental rats. However, compared to rats given only CdCl2, co-administration of CdCl2 and gallic acid at a dose of 20 mg/kg bwt markedly (p < 0.05) raised the activities of SOD and catalase as well as GSH level.
Brain cerebrum levels of antioxidants and MDA in control and treated groups. (a) SOD, (b) CAT, (c) GSH, and (d) MDA between the control and treated groups. The data is represented as mean ± SD (n = 5). #Significant at p < 0.05 compared to the negative control; *Significant at p < 0.05 compared to the CdCl2 alone.
Gallic acid reversed the inhibition of brain activities of acetylcholinesterase and butyrylcholinesterase in rats administered with CdCl2
Figure 2 shows the impact of gallic acid on the brain activities of AChE and BChE in the cerebrum of rats given CdCl2. Compared to control rats, CdCl2 markedly (p < 0.05) inhibited AChE and BChE activities in the cerebrum of Wistar rats (Fig. 2). However, the activities of both enzymes were significantly decreased (p < 0.05) when CdCl2 and gallic acid were administered together.
Gallic acid reverses the inhibition of (a) acetylcholinesterase (AChE) and (b) butyrylcholinesterase (BChE) of rats administered with distilled water, CdCl2 alone, gallic acid alone, and CdCl2 + gallic acid (20 mg/kg). The data is represented as mean ± SD (n = 5). #Significant at p < 0.05 compared to the control; *Significant at p < 0.05 compared to the CdCl2 only group.
Gallic acid reverses brain levels of serotonin and dopamine in rats exposed to CdCl2
The effect of gallic acid on brain neurotransmitter levels of serotonin (Fig. 3) and dopamine (Fig. 4) in the cerebrum of rats administered CdCl2. CdCl2 alone significantly (p < 0.05) reduced serotonin levels and increased dopamine levels in the brains of Wistar rats compared to control rats. On the contrary, the co-administration of gallic acid with CdCl2 significantly (p < 0.05) prevent such alterations in the level of neurotransmitters. Furthermore, in rats administered only gallic acid, serotonin levels increased and dopamine decreased compared to CdCl2 alone (p < 0.05).
Gallic acid reverses the levels of serotonin in the cerebrum of rats administered with distilled water, CdCl2 alone, gallic acid alone, and CdCl2 + gallic acid (20 mg/kg). The data is represented as mean ± SD (n = 5). #Significant at p < 0.05 compared to the control; *Significant at p < 0.05 compared to the CdCl2 only group.
Gallic acid reverses the levels of dopamine in the cerebrum of rats administered with distilled water, CdCl2 alone, gallic acid alone, and CdCl2 + gallic acid (20 mg/kg). The data is represented as mean ± SD (n = 5). #Significant at p < 0.05 compared to the control; *Significant at p < 0.05 compared to the CdCl2 only group.
Gallic acid protected against CdCl2-induced neuro inflammation
The gallic acid effects on MPO, NO, and interleukin-6 (IL-6) brain activity in the cerebrum of rats receiving CdCl2 are shown in Figs. 5, 6, and 7. CdCl2 alone significantly (p < 0.05) raised the levels of MPO (Fig. 5), NO (Fig. 6), and IL-6 (Fig. 7) in comparison to the control. Co-administration of CdCl2 and gallic acid decreased NO and IL-6 levels as well as MPO activity in comparison to rats exposed to CdCl2 alone.
Effect of gallic acid on membrane bound ATPases in rat brain
Changes in ATPase enzyme activity (Na+/K+-ATPase) in the cerebrum of control and experimental rats are depicted in Fig. 8. Compared to control rats, CdCl2 alone rats had a significant decrease (p < 0.05) in the activity of the ATPase enzyme. Compared to cadmium alone, gallic acid co-administration significantly increased (p < 0.05) the activity of ATPase enzymes in the brain.
Impact of gallic acid on brain of rats with CdCl2-induced neurotoxicity
Figure 9 shows the histological studies of the cerebrum of experimental animals after the administration of CdCl2 + gallic acid. In contrast to the normal architecture displayed by the cerebrum of normal and gallic acid control rats, the cerebrum of CdCl2-induced rats had severe vacuolation of purkinje cell layer, optical empty spaces due to cell necrosis, severe separation of purkinje cell layer from granular layer, severe hemorrhage, and cell degeneration. The co-administration of CdCl2 with gallic acid decreased the occurrence of these changes in the cerebrum of rats and revealed near-normal architecture comparable to the CdCl2 alone rats.
Impact of gallic acid on the cerebrum of CdCl2-induced neurotoxic rats (H & E, 200X). Group A (Control rats), Group B (cadmium chloride-treated rats), Group C (CdCl2 + GA-treated rats), Group D (gallic acid-treated rats) (A) Control showing normal granular layer, molecular layer, purkinje cell layer, blood vessels as well as while matter. (B) CdCl2 group showing severe vacuolation of purkinje cell layer, optical empty spaces due to necrosis (arrow), severe separation of purkinje cell layer from granular layer, severe hemorrhage of white matter; (C) CdCl2 + GA group showing vacuolated purkinje cell layer (black arrow), mild separation of purkinje cell layer; (D) GA group reveals normal granular layer, molecular layer, vacuolated purkinje cell layer (black arrow).
Discussion
According to recent research, Cd exposure in Wistar rats weakening antioxidant defense mechanisms, which messes up cellular redox and causes oxidative stress. During oxidative stress, free radicals are produced in large quantities, causing a reduction in antioxidant enzymes like superoxide dismutase and catalase32, which may have helped control ROS in the brain33. However, the brain can suffer harm if ROS is generated by interacting with the lipids, carbohydrates, proteins, and DNA present in the brain31. This study suggests that CdCl2 toxicity is caused by oxidative stress, which interacts with various brain cells and tissues. As a result, CdCl2-induced oxidative stress in rat brains, leading to decreased SOD and catalase activities, which is a sign of oxidative damage.
Shukla and Chandra30 and Ojo et al.13,34 found a similar decrease in catalase and SOD activities in rat brains after CdCl2 intoxication. The restoration of SOD and catalase activities in the brains of rats suggests that gallic acid has protective potential by reducing lipid peroxidation in brain tissues. Meanwhile, a drop in GSH levels has been noted in some earlier studies. Due to the excessive availability of the lipid peroxidation product (MDA) and inhibition of enzymes by CdCl2 metabolites, the level of GSH was lower in the current study. This could be due to its ability to conjugate electrophilic metabolites of CdCl2. Gallic acid, on the other hand, may help in the management of ROS in the brain as a neuroprotective agent due to its ability to prevent CdCl2-induced inhibition of GSH in the brain. The buildup of Cd in brain tissue, which depletes the GSH pool, may be the cause of the decrease in antioxidant enzyme levels observed in this study35. In addition to the binding of Cd to the sulfhydryl (-SH) group of the oxidative enzymes and inhibiting them, GSH depletion also renders the antioxidant enzymes inactive36. The current findings are consistent with those of Elkhadrag et al. and Ichipi Ifukor et al.37,38. Rutin, a well-known antioxidant, has been shown to have a similar effect on these antioxidant parameters39.
The neurotoxin potency of Cd has been demonstrated in both in vitro and in vivo studies40,41. Animals exposed to Cd have been found to have altered catecholaminergic and serotoninergic transmission42,43. To determine the neurotoxic effects of heavy metals, it is imperative to investigate the activities of brain enzymes like AChE and BChE. According to several studies44, AChE activity in the brain is believed to decline as a result of free radical production in the brain. Acetylcholine accumulation, which results in cholinergic hyperactivity, convulsions, and epileptic status, has been shown to be caused by decreased AChE45. Although it is unclear how much it directly contributes, decreased levels of AChE in the brain could be one of the indicators of Cd-induced injury because they are a key regulator of behavioral processes. The fact that CdCl2 inhibited the activities of butyrylcholinesterase and acetylcholinesterase in the brains of rats in this study indicates that acetylcholine had accumulated due to disruptions cholinergic transmission. This result is consistent with Pari and Murugavel's report on the effect of diallyl tetrasulfide, which is commonly found in garlic, on AChE46.
The most common neurotransmitter in the central nervous system, serotonin (5-HT), is essential for memory and learning47. On the other hand, tryptophan hydroxylase is distributed similarly to serotonin (5-HT) in the hypothalamus, the midbrain, and the limbic system of the brain48,49,50. In this study, rats treated with cadmium alone had considerably lower levels of 5-HT in their brains. By inhibiting the oxidative metabolism of serotonergic neurons, cadmium prevents tryptophan hydroxylase activity, which is essential for the conversion of tryptophan to 5-HT51. Our results are in line with those of Das et al.52, who discovered that rats given CdCl2 had markedly lower levels of 5-HT in their brains. In the current study, brain catecholamine levels (dopamine) increased in rats administered cadmium. It has been proposed that it causes changes in neurochemistry as a result of oxidative damage to brain tissue, which may be responsible for its toxic action53,54. Cd also promotes lipid peroxidation by increasing the production of superoxide anions55, which act as primary oxidant. This process can cause direct cell damage by destroying membranes and indirect cell damage by producing reactive carbonyl products56. Cd-induced lipid peroxidation is the cause of membrane damage that leads to neuronal dysfunction, resulting in decreased dopamine uptake in the brain57. Our findings are consistent with30, who found that low-dose chronic cadmium treatment markedly increased dopamine levels in rats. Additionally58, found that low-dose chronic cadmium treatment elevated dopamine levels in rats.
Due to the presence of free fatty acids in the brain, it is extremely prone to lipid peroxidation59. The increase in MDA levels caused by CdCl2 in the current study suggests that the brains of rats may experience cellular damage and dysfunction as a result of excessive ROS generation60. This supports the hypothesis that a decrease in glutathione levels is the first sign of oxidative stress. Rotimi et al.14 made a similar finding of elevated MDA levels in CdCl2-induced rats. On the other hand, gallic acid's capacity to restore MDA levels that are comparable to those of the relevant controls may be connected to its anti-peroxidative properties.
One of the defenders against neurotoxic assaults on a host's nervous system is neuroinflammation. As a promising clinical target, neuroinflammation is also a frequent pathological outcome or etiology of neurodegeneration and neurological disorders61. Therefore, the detection of specific neuroinflammation markers was crucial in this study. For example, the pathogenesis of inflammatory disorders and the maintenance of neural tissue homeostasis depend on the cytokine interleukin-6 (IL-6)62. Nitric oxide enhances cerebral blood flow while acting as a retrogressive neurotransmitter in synapses and is crucial for intracellular signaling in neurons63. Myeloperoxidase is a therapeutic target for oxidative stress and neuroinflammation-causing pathological processes64. The rise in IL-6 levels in the brains of rats suggests that it interferes with the physiological processes associated with neuroinflammation. This justify the role of IL-6 in the pathogenesis of neurodegenerative diseases. In this study, nitric oxide levels were higher than normal in the brains of rats due to CdCl2, which may also have interfered with intracellular signaling in the brain. Reduced antioxidant status with elevated oxidative stress was associated with increased MPO activity in the rat brain. Gallic acid, on the other hand, has the potential to be an anti-inflammatory agent in inflammation-induced brain diseases, as evidenced by its ability to reverse the increase in NO and MPO induced by CdCl2.
The production of metabolic energy, the uptake and release of catecholamines and serotonin, as well as neural excitability, are all regulated by the enzyme Na+/K+-ATPase65,66. Furthermore, Mg2+ -ATPase is responsible for keeping intracellular Mg2+ levels in the brain at a high level, where changes can affect the rate of protein synthesis and cell growth67. In the nervous system, Ca2+ acts as a second messenger. Numerous pathological lesions in the brain are caused by variations in Ca2+ levels68 . The Ca2 + ATPase activity in the cell membrane is also inhibited by the Ca2+ overload caused by Cd, which ultimately results in irreversible cell death. Exposure to Cd affects the activities of these ATPase enzymes17,43,69,70, indicating changes in membrane and neurotransmitter functions. Compared to control rats, CdCl2 alone showed a significant decrease in ATPase enzyme activity in the brains of experimental animals. ATPase activity can be reduced due to the formation of Cd-ATPase complexes through the enzyme’s SH group and/or increased oxidative stress46. Co-administration of gallic acid with Cd significantly increased the activity of ATPase enzymes in the brain compared to cadmium alone.
Significant histopathological changes in brain tissue have been widely associated with heavy metals71. According to histological examination of brain tissue, Cd intoxication causes abnormal structural changes in brain tissue, including apoptosis, nuclear vacuolization, and inflammatory lymphocytic changes. Cd alters the cortical micro-and macrostructures once it enters the brain. Cd destroys glial and neuronal cells in the hippocampus’s white matter72. Cadmium causes severe damage to the brain, including encephalopathy and hemorrhage. These changes are neuropathological and neurochemical. The cortical neuroglia, pyramidal, and granule cells are also altered by Cd exposure73. Additionally, Cd affects the structure of nerve cells and parenchyma, impairing attention, memory, and olfactory functions as well as hypernociception74. According to a histopathological assessment of the cerebrum, the intoxication with cadmium led to abnormal ultra-structural changes, including severe vacuolation of purkinje cell layer, optical empty spaces due to cell necrosis, severe separation of purkinje cell layer from granular layer, severe hemorrhage, and cell degeneration. The observed pathological damage caused by CdCl2-induced rats treated with gallic acid showed a significant recovery, indicating that gallic acid is capable of abating the neuronal impairment caused by cadmium. As a result, it might be hypothesized that gallic acid could prevent brain damage caused by Cd.
Our obtained data showed that CdCl2 induced oxidative stress and neuroinflammation in the brains of Wistar rats. However, gallic acid offered neuroprotective effects on CdCl2-induced toxicity in the brain, indicating its antioxidant and therapeutic potentials against oxidative stress and neuro-inflammation in the brain of rats exposed to CdCl2. Our results suggested that gallic acid showed a profound improvement in neurotransmitter levels.
Materials and methods
Chemicals
Gallic acid (GA) was a product of SantaCruz Biotechnology, Heidelberg, Germany. ELISA kits for serotonin, dopamine, and interleukin-6 were purchased from Elabscience, Houston, Texas, USA. All other chemicals were of analytical grade.
Animals
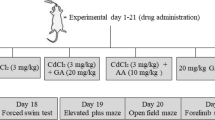
For this experiment, twenty (20) Wistar rats weighing between 170 and 200 g were used. The animals were acquired from Landmark University's Biochemistry Department in Kwara State, Nigeria. Throughout the 21-day acclimatization period and the experimental period, they were kept at room temperature with unlimited access to food and water (21 days).
Ethical approval
All experimental rats used in this study were handled in accordance with the rules and regulations established for animal management in research, as outlined in NIH Publications No. 80-23 revised, 1996. The Animal Care and Use Ethical Committee at Landmark University, Nigeria (LUAC/2020/0052B) confirmed and approved that the experimental treatment of the rats is in accordance with ARRIVE guidelines. Furthermore, all methods/experimental protocol/s were approved by the institution's ethical committee and ARRIVE guidelines.
Animal groupings and experimental procedures
The rats were divided into four groups (n = 5) at random. The negative control group was given distilled water only for the duration of the study. Group 2 was the positive control, receiving only CdCl2 (5 mg/kg/day) throughout the study. Group 3 received only 20 mg/kg of gallic acid per day, whereas Group 4 received 20 mg/kg of gallic acid + 5 mg/kg of CdCl2. The doses of cadmium and gallic acid were chosen based on previous studies6,75 for cadmium and13,76 for gallic acid. Gallic acid was said to be safe at a daily dose of 20 mg/kg body weight76, but it has been demonstrated that the cadmium dose chosen causes significant oxidative stress in various tissues34,77.
Serum and organ collection
Animals were sacrificed under halothane anesthesia by cervical dislocation twenty-four hours after the last administration. Cardiovascular puncture was used to quickly collect blood into a plain bottle, which was then spun at 3000 rpm for 10 min to obtain serum, which was used to assess biochemical indices. Quickly removed from the body, the brain was rinsed in ice-cold normal saline and the cerebrum was homogenized in 0.1 M phosphate buffer (1:10 w/v) (pH 7.4). The brains were sectioned and the cerebrum were separated. At 3000 rpm, the homogenates were centrifuged. The obtained clear supernatant was used for various biochemical assays.
Biochemical analyses
Using Ellman's method78, the activities of acetylcholinesterase (AChE) and butyrylcholinesterase were assessed. The TBA Reactive Substances (TBARS) concentration was measured using the Buege and Aust method79. The Na+/K+ ATPase's activity was assessed by Bonting80. The technique outlined by Misra and Fridovich81 was used to test the superoxide dismutase activity. The catalase activity was tested using the procedure outlined by Aebi82. The method outlined by Ellman83 was used to measure the level of reduced glutathione (GSH). The assay for myeloperoxidase activity was carried out using the technique described by Granell et al.84. The Griess reaction was used to calculate the nitric oxide level85. The levels of IL-6, dopamine, and serotonin were estimated using rat specific enzyme linked immunosorbent assay (ELISA) kits (ElabScience, USA) according to the manufacturer’s instructions.
Histopathological investigation
Animal brain tissues were used for histopathological research. The tissues underwent routine processing before being formalin-fixed in 10% formalin and embedded in paraffin wax. Hematoxylin and eosin (H and E) were used to stain the cerebrum sections, which were of 4 µm thick, on glass slides. A light microscope was used to examine the slides, and magnified images (200X) of the tissue structures were recorded86.
Data analysis
Data analysis was performed using one-way analysis of variance (ANOVA) on GraphPad Prism 9.0, version (GraphPad software, Inc, San Diego, USA). All results were expressed as mean ± standard deviation (SD). Statistical significance at p < 0.05 was determined by using Tukey's post hoc multiple comparisons test.
Data availability
Data is available on reasonable request from the corresponding author.
References
ATSDR (Agency for Toxic Substances and Disease Registry). U.S. Toxicological Profile for Cadmium (Department of Health and Humans Services, Public Health Service, Centers for Disease Control, 2005).
Ali, H., Khan, E. & Ialhi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 1–14 (2019).
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A. & Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 17, 3782 (2020).
Satarug, S., Garrett, S. H., Sens, M. A. & Sens, D. A. Cadmium, environmental exposure and health outcomes. Environ. Health Perspect. 118, 182–190 (2010).
Renugadevi, J. & Miltonprabu, S. Protective role of alpha tocopherol and ascorbic acid against cadmium induced neurotoxicity in rats. Int. J. Med. Sci. 2, 11–17 (2009).
El-demerdash, F. M., Yousef, M. I., Kedwany, F. S. & Baghdadi, H. H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2, 11–17 (2004).
González-Trujano, E. & Navarrete, A. Effect of zinc on the cadmium acute intoxication in the gastric injury induced in rats. Rev. Latinoamer. Quím 39(1–2), 45–54 (2011).
Branca, J. J., Morucci, G. & Pacini, A. Cadmium-induced neurotoxicity: Still much ado. Neural Regen. Res. 13(11), 1879 (2018).
Limón-Pacheco, J. & Gonsebatt, M. E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Res. 674(1–2), 137–147 (2009).
Ajiboye, B. O. et al. Inhibitory effect of ethyl acetate fraction of Solanum macrocarpon L. leaves on cholinergic, monoaminergic and purinergic enzyme activities. Food Biochem. 42(6), e12643. https://doi.org/10.1111/jfbc.12643 (2018).
Ajiboye, B. O., Ojo, O. A., Okesola, M. A., Oyinloye, B. E. & Kappo, A. P. Ethyl acetate leaf fraction of Cnidoscolus aconitifolius (Mill.) I. M. Johnst: Antioxidant potential, inhibitory activities of key enzymes on carbohydrate metabolism, cholinergic, monoaminergic, purinergic and chemical fingerprinting. Int. J. Food Crops 21(1), 1697–1715 (2018).
Oboh, G. et al. Rutin alleviates cadmium-induced neurotoxicity in Wistar rats: Involvement of modulation of nucleotide-degrading enzymes and monoamine oxidase. Metab. Brain Dis. 34, 1181–1190 (2019).
Shukla, G. S. & Chandra, S. V. Concurrent exposure to lead, manganese, and cadmium and their distribution to various brain regions, liver, kidney, and testis of growing rats. Arch. Environ. Contam. Toxicol. 16, 303–310. https://doi.org/10.1007/BF01054947 (1987).
Chen, L. et al. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 50, 624–632 (2011).
Ojo, O. A. et al. Gallic acid protects against cadmium chloride-induced alterations in Wistar rats via the antioxidant defense mechanism. J. Pharm. Pharmacogn. Res 9(5), 668–676 (2021).
Rotimi, D. et al. Protective impacts of gallic acid against cadmium-induced oxidative toxicity in the ovary of rats. Comp. Clin. Pathol. 30(3), 453–460. https://doi.org/10.1007/s00580-021-03237-w (2021).
EL-missiry, M. A. & Shalaby, F. Role of beta-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. J. Biochem. Mol. Toxicol. 14, 238–243 (2000).
Nayeem, N., Asdaq, S. M. B., Salem, H. & Ahel-Alfqy, S. Gallic acid: A promising lead molecule for drug development. J. Appl. Pharm. 8(2), 213–218. https://doi.org/10.4172/1920-4159.1000213 (2016).
Kahkeshani, N. et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran J. Basic Med. Sci. 22(3), 225–237. https://doi.org/10.22038/ijbms.2019.32806.7897 (2019).
Gao, J., Hu, J., Hu, D. & Yang, X. A role of gallic acid in oxidative damage diseases. Nat. Prod. Commun. https://doi.org/10.1177/1934578X19874174 (2019).
Wu, X. C., Yu, B. T., Hou, A. L., Hu, T. T. & Lu, G. Q. Study on stability of gallic acid. Med. J. Natl. Def. Forces Southwest China 16, 484–485 (2006).
Mansouri, M. T. et al. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 138, 1028–1033 (2013).
Sarkaki, A. et al. Gallic acid improved behavior, brain electrophysiology, and inflammation in a rat model of traumatic brain injury. Can. J. Physiol. Pharmacol. 93, 687–694 (2015).
Inoue, M. et al. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 18(11), 1526–1530 (1995).
Ohno, Y. et al. Induction of apoptosis by gallic acid in lung cancer cells. Anticancer Drugs 10(9), 845–851 (1999).
Priscilla, D. H. & Prince, P. S. M. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 179(2–3), 118–124 (2009).
Patel, S. S. & Goyal, R. K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacogn. Res. 3(4), 239. https://doi.org/10.4103/0974-8490.89743 (2011).
Rasool, M. K. et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 62(5), 638–643 (2010).
Ohno, T., Inoue, M. & Ogihara, Y. Cytotoxic activity of gallic acid against liver metastasis of mastocytoma cells P-815. Anticancer. Res. 21(6A), 3875–3880 (2001).
Kim, M. J. et al. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr Food Res. 55(12), 1798–1808 (2011).
Kroes, B. V., Van den Berg, A., Van Ufford, H. Q., Van Dijk, H. & Labadie, R. Anti-inflammatory activity of gallic acid. Planta Med. 58(6), 499–504. https://doi.org/10.1055/s-2006-961535 (1992).
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. & Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 5, 9–19 (2012).
Kaygusuzoglu, E. et al. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in femalewistar rats. Biomed. Pharmacother. 102, 517–530 (2018).
Ojo, O. A., Oyinloye, B. E., Ajiboye, B. O. & Onikanni, S. A. Neuroprotective mechanism of ethanolic extract of Irvingia gabonensis stem bark against cadmium-induced neurotoxicity in rats. Br. J. Med. Med. Res. 4(36), 5793–5805 (2014).
Onyema, O. O., Farombi, E. O., Emerole, G. O., Ukoha, A. I. & Onyeze, G. O. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J. Biochem. Biophys. 43, 20–24 (2006).
Renugadevi, J. & Prabu, S. M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 256, 128–134. https://doi.org/10.1016/j.tox.2008.11.012 (2009).
Elkhadragy, M. F., Al-Olayan, E. M., Al-Amiery, A. A. & Abdel Moneim, A. E. Protective effects of Fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol. Trace Elem. Res. 181, 378–387. https://doi.org/10.1007/s12011-017-1062-7 (2018).
Ichipi-Ifukor, P. C., Asagba, S. O., Nwose, C., Mordi, J. C. & Oyem, J. C. Palm oil extracts protected against cadmium chloride poisoning via inhibition of oxidative stress in rats. Bull. Natl. Res. Cent. 46(1), 1–11 (2022).
Mostafa, D. G., Khaleel, E. F., Badi, R. M., Abdel-Aleem, G. A. & Abdeen, H. M. Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol. Res. 41(7), 594–608 (2019).
Webster, W. S. & Valois, A. A. The toxic effect of cadmium on the neonatal mouse CNS. J. Neuropathol. Exp. Neurol. 40, 247–257 (1981).
Kabeer, I. A., Rajender, R. J. & Desaiah, D. Protection against cadmium toxicity and enzyme inhibition by dithiothreitol. Cell Biochem. Funct. 7, 185–192 (1989).
Gupta, A., Gupta, A. & Shukla, S. G. Neurochemical changes in developing rat brain after pre- and postnatal cadmium exposure. Bull. Environ. Contam. Toxicol. 51, 12–17 (1993).
Antonio, M. T., Corredor, L. & Leret, M. L. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol. Lett. 143, 331–340 (2003).
Tsakiris, S., Angelogianni, P., Schulpis, K. H. & Stavridis, J. C. Protective effect of l-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin. Biochem. 33, 103–106 (2000).
Olney, J. W., Collins, R. C. & Sloviter, R. S. Exotoxic mechanisms of epileptic brain damage. Adv. Neurol. 44, 857–877 (1986).
Pari, L. & Murugavel, P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology 234(1–2), 44–50 (2007).
Butzlaff, M., Ponimaskin, E., Butzlaff, M. & Ponimaskin, E. The role of serotonin receptors in Alzheimer’s disease. Opera Med. Physiol. 1, 91–100 (2016).
Ney, D. M. et al. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol. Genet. Metab. 121, 96–103 (2017).
Fernstrom, J. D. & Fernstrom, M. H. Tyrosine, phenylalanine, and catechol- amine synthesis and function in the brain. J. Nutr. 137, 539S-1547S (2007).
Haider, S., Khaliq, S., Ahmed, S. P. & Haleem, D. J. Long-term tryptophan administration enhances cognitive performance and increases 5HT metabolism in the hippocampus of female rats. Amino Acids 31, 421–425 (2006).
Batool, Z. et al. Attenuation of cadmium-induced decline in spatial, habituation and recognition memory by long-term administration of almond and walnut supplementation: Role of cholinergic function. Pak. J. Pharm. Sci. 30, 273–279 (2017).
Das, K., Das, P., Dasgupta, S. & Dey, C. Serotonergic-cholinergic neurotransrnitters’ functionin brain during cadmium exposure in proteinrestricted rat. Biol. Trace Elem. Res. 36, 119–127 (1993).
Wang, B. & Du, Y. Cadmium and its neurotoxic effects. Oxid. Med. Cell Longev. 2013, 898034 (2013).
Wang, H., Zhang, L., Abel, G. M., Storm, D. R. & Xia, Z. Cadmium exposure impairs cognition and olfactory memory in male C57BL/6 mice. Toxicol. Sci. 161, 87–102 (2018).
Wright, R. O. & Baccarelli, A. Metals and neurotoxicology. J Nutr 137, 2809–2813 (2007).
Esterbauer, H., Zollner, H. & Schaur, R. In Lipidoxidation (Vigo, C., Pel Frey, B. A., eds) 239–283 (CRC Press, 1990).
Rajanna, B., Hobson, M., Boykin, M. & Chetty, C. Effects of chronic treatment with cadmium on ATPases, uptake of catecholamines and lipid peroxidation in rat brain synaptosomes. Ecotoxicol. Environ. Saf. 20, 36–41 (1990).
Zhai, Q., Narbad, A. & Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 7, 552–571 (2015).
Yan, H. F. et al. Ferroptosis: Mechanisms and links with dieases. Signal Transduct. Target. Ther. 6, 49 (2021).
Ajayi, A. M., Ben-Azu, B., Godson, J. C. & Umukoro, S. Effect of spondias mombin fruit extract on scopolamine-induced memory impairment and oxidative stress in mice brain. J. Herbs. Spices Med. Plants 27, 24–36 (2020).
Mishra A, et al. (2021) Neuroinflammation in Neurological Disorders: Pharmacotherapeutic Targets from Bench to Bedside. Springer, Berlin
Rothaug, M., Becker-Pauly, C. & Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 1863, 1218–1227 (2016).
Picón-Pagès, P., Garcia-Buendia, J. & Muñoz, F. J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta 1865, 1949–1967 (2019).
Chen, S., Chen, H., Du, Q. & Shen, J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 11, 433 (2020).
Bogdanski, D. F., Tissuri, A. & Brodie, B. B. Role of sodium, potassium, ouabain and reserpine in uptake, storage and metabolism of biogenic amines in synaptosomes. Life Sci. 7, 419–428 (1968).
Mata, M. et al. Activity-dependent energy metabolism in rat posterior pituitary, primarily reflects sodium pump activity. J. Neurochem. 34, 214–215 (1980).
Sanui, H. & Rubin, H. The role of magnesium in cell proliferation and transformation. In Ions, Cell Proliferation and Cancer (eds Boynton, A. L. et al.) 517–537 (Academic Press, 1982).
Repetto, M. Toxicologia Fundamental (Díaz de Santos Editions, 1997).
Rajanna, B., Hobson, M., Boykin, M. & Chetty, C. S. Effects of chronic treatment with cadmium on ATPases, uptake of catecholamines and lipid peroxidation in rat brain synaptosomes. Ecotoxicol. Environ. Saf. 20, 36–41 (1990).
Carfagna, M. A., Ponsler, G. D. & Muhoberac, B. B. Inhibition of ATPase activity in rat synaptic plasma membranes by simultaneous exposure to metals. Chem. Biol. Interact. 100, 53–65 (1996).
Al-Quraishy, S., Dkhil, M. A., Ibrahim, S. R. & Abdel Moneim, A. E. Neuroprotective potential of Indigofera oblongifolia leaf methanolic extract against lead acetate-induced neurotoxicity. Neural Regen. Res. 11, 1797–1803. https://doi.org/10.4103/1673-5374.194749 (2016).
El-Tarras, A. E. S., Attia, H. F., Soliman, M. M., El Awady, M. A. & Amin, A. A. Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 29, 398–407. https://doi.org/10.1177/0394632016651447 (2016).
Afifi, O. K. & Embaby, A. S. Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. J. Microsc. Ultrastruct. 4, 36–45. https://doi.org/10.1016/j.jmau.2015.10.001 (2016).
Wang, B. & Du, Y. Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013, 12. https://doi.org/10.1155/2013/898034 (2013).
Shagirtha, K., Muthumani, M. & Milton Prabu, S. Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Euro Rev. Med. Pharmacol. Sci. 15, 1039–1050 (2011).
Ola-Davies, O. E. & Olukole, S. G. Gallic acid protects against bisphenol A-induced alterations in the cardiorenal system of Wistar rats through the antioxidant defense mechanism. Biomed. Pharmacother. 107, 1786–1794 (2018).
Ojo, O. A., Ajiboye, B. O., Oyinloye, B. E., Ojo, A. B. & Olarewaju, O. I. Protective effect of Irvingia gabonensis stem bark extract on cadmium-induced nephrotoxicity in rats. Interdiscip. Toxicol. 7(4), 208–214 (2014).
Ellman, G. L., Courtney, K. D., Andres, V. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7(2), 88–95 (1961).
Buege, J. A. & Aust, S. D. Biomembranes-Part C: Biological oxidations. Methods Enzymol. 52, 302–310 (1978).
Bonting, S. L. Presence of enzyme system in mammalian tissues. In Membrane and Ion Transport (ed. Bilter, E. E.) 257–263 (Wiley Inter Science, 1970).
Misra, H. P. & Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170–3175 (1972).
Aebi, H. Catalase estimation. In Methods of Enzymatic Analysis (ed. Bilter, E. E.) 673–684 (Verlag Chemie/Academic Press Inc, 1974).
Ellman, G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 302–310 (1959).
Granell, S. et al. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 31, 525–530 (2003).
Green, L. C. et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126, 131–138 (1982).
Drury, R. A., Wallington, E. A. & Cancerson, R. Carlton’s Histopathological Techniques 4th edn. (Oxford University Press, 1976).
Author information
Authors and Affiliations
Contributions
O.A.O., D.E.R., and A.B.O. conceptualized and designed the research study, D.E.R. and O.A.O. performed the study. O.A.O., D.E.R., A.B.O., A.D.O., and B.O.A. generated and curated the data. D.E.R., A.D.O., A.B.O., and O.A.O. wrote the first draft of the manuscript; O.A.O., A.B.O., A.D.O., and B.O.A. revised the manuscript for intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojo, O.A., Rotimi, D.E., Ojo, A.B. et al. Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats. Sci Rep 13, 1577 (2023). https://doi.org/10.1038/s41598-023-28893-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28893-6
- Springer Nature Limited