Abstract
Purpose
The polyphenolic molecule gallic acid, which functions as an antioxidant by removing free radicals and chelating metals, has garnered a lot of attention. In animal studies, cadmium exposure has been linked to neurotoxic effects and cognitive impairment. In this context, the present study aimed at investigating the effect of gallic acid on the neurochemical change and cognitive impairment in rats exposed to cadmium.
Methods
Twenty-four Wistar rats were exposed to cadmium (5 mg/kg) for 21 days and thereafter treated with gallic acid (25 and 50 mg/kg). After the treatment period, the rats were subjected to neurobehavioral tests before being sacrificed under mild anesthesia. The brain was removed and dissected into the cortex and hippocampus, and the homogenates of these brain sections were prepared for biochemical analyses.
Results
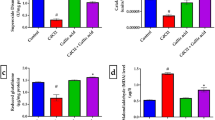
Findings revealed a cognitive impairment due to cadmium exposure, and this was ameliorated by treatment with gallic acid. In addition to disruption of redox homeostasis, alteration in the enzyme activity of cholinergic and purinergic systems was observed in rats exposed to cadmium. However, treatment with gallic acid restored redox balance by enhancing the activity of some antioxidant enzymes and reducing the concentration of oxidative stress markers. Also, treatment with gallic acid modulated the enzyme activity of cholinergic and purinergic systems.
Conclusion
Thus, gallic acid treatment reversed cognitive impairment, and alteration to the cholinergic and purinergic systems caused by subchronic cadmium exposure in rats, possibly via an antioxidant mechanism.






Similar content being viewed by others
Data availability
Data is available on request.
References
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021;12:643972.
Nemmiche S, Chabane-Sari D, Guiraud P. Role of α-tocopherol in cadmium-induced oxidative stress in Wistar rat’s blood, liver and brain. Chem-Biol Interact. 2007;170(3):221–30.
Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23(5):783–92.
Unsal V, Dalkıran T, Çiçek M, Kölükçü E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv Pharm Bull. 2020;10(2):184.
Méndez-Armenta M, Rios C. Cadmium neurotoxicity. Environ Toxicol Pharmacol. 2007;23(3):350–8.
Branca JJ, Maresca M, Morucci G, Mello T, Becatti M, Pazzagli L, Colzi I, Gonnelli C, Carrino D, Paternostro F, Nicoletti C. Effects of cadmium on ZO-1 tight junction integrity of the blood brain barrier. Int J Mol Sci. 2019;20(23):6010.
Al Omairi NE, Radwan OK, Alzahrani YA, Kassab RB. Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab Brain Dis. 2018;33:1121–30.
Bakulski KM, Hu H, Park SK. Lead, cadmium and Alzheimer’s disease. Genet Neurol Behav Diet Dement. 2020;1:813–30.
da Costa P, Gonçalves JF, Baldissarelli J, Mann TR, Abdalla FH, Fiorenza AM, da Rosa MM, Carvalho FB, Gutierres JM, de Andrade CM, Rubin MA. Curcumin attenuates memory deficits and the impairment of cholinergic and purinergic signaling in rats chronically exposed to cadmium. Environ Toxicol. 2017;32(1):70–83.
Oboh G, Adebayo AA, Ademosun AO, Olowokere OG. Rutin alleviates cadmium-induced neurotoxicity in Wistar rats: involvement of modulation of nucleotide-degrading enzymes and monoamine oxidase. Metab Brain Dis. 2019;34(4):1181–90.
Ferreira-Vieira HT, Guimaraes MI, Silva RF, Ribeiro MF. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–15.
Flora SJS, Pachauri V. Chelation in metal intoxication. Intl J Environ Res Public Health. 2010;7:2745–88.
Szwajgier D, Borowiec K, Pustelniak K. The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients. 2017;9(5):477.
Del Bo’ C, Bernardi S, Marino M, Porrini M, Tucci M, Guglielmetti S, Cherubini A, Carrieri B, Kirkup B, Kroon P, Zamora-Ros R. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern?. Nutrients. 2019;11(6):1355.
Liu J, He Z, Ma N, Chen ZY. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. J Agric Food Chem. 2019;68(1):33–47.
Maurya DK, Nandakumar N, Devasagayam TP. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J Clin Biochem Nutr. 2010;48(1):85–90.
Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5(35):27540–57.
Perazzoli MR, Perondi CK, Baratto CM, Winter E, Creczynski-Pasa TB, Locatelli C. Gallic acid and dodecyl gallate prevents carbon tetrachloride-induced acute and chronic hepatotoxicity by enhancing hepatic antioxidant status and increasing p53 expression. Biol Pharm Bull. 2017;40(4):425–34.
Lee CY, Su CH, Tsai PK, Yang ML, Ho YC, Lee SS, Chen CH, Chen WY, Lin ML, Chen CJ, Chian CY. Cadmium nitrate-induced neuronal apoptosis is protected by N-acetyl-l-cysteine via reducing reactive oxygen species generation and mitochondria dysfunction. Biomed Pharmacother. 2018;108:448–56.
Tsai CY, Fang C, Wu JC, Wu CJ, Dai KY, Chen SM. Neuroinflammation and microglial activation at rostral ventrolateral medulla underpin cadmium-induced cardiovascular dysregulation in rats. J Inflamm Res. 2021;14:3863.
Abdel-Aleem GA, Khaleel EF. Rutin hydrate ameliorates cadmium chloride-induced spatial memory loss and neural apoptosis in rats by enhancing levels of acetylcholine, inhibiting JNK and ERK1/2 activation and activating mTOR signalling. Arch Physiol Biochem. 2018;124(4):367–77.
Mohamed HM, Abd El-Twab SM. Gallic acid attenuates chromium-induced thyroid dysfunction by modulating antioxidant status and inflammatory cytokines. Environ Toxicol Pharmacol. 2016;48:225–36.
Adeniyi PA, Omatsuli EP, Akinyemi AJ, Ishola AO. Caffeine plus nicotine improves motor function, spatial and non-spatial working memory and functional indices in BALB/c male mice. Pathophysiology. 2016;23(4):251–8.
Ademosun AO, Adebayo AA, Popoola TV, Oboh G. Shaddock (Citrus maxima) peels extract restores cognitive function, cholinergic and purinergic enzyme systems in scopolamine-induced amnesic rats. Drug Chem Toxicol. 2022;45(3):1073–80.
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95.
Schetinger MR, Porto NM, Moretto MB, Morsch VM, da Rocha JB, Vieira V, Moro F, Neis RT, Bittencourt S, Bonacorso HG, Zanatta N. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem Res. 2000;25(7):949–55.
Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res. 2007;631(1):55–61.
Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5.
Claiborne A. Catalase activity. In: Greenwald RA, editor. Handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1985. p. 283–4.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Seok H, Lee M, Shin E, Yun MR, Lee YH, Moon JH, Kim E, Lee PH, Lee BW, Kang ES, Lee HC. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep. 2019;9(1):1–10.
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M. Oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2016;53(6):4094–125.
Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. 2020;37(1):113–39.
Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44(10):1125–71.
Agostini JF, Dal Santo G, Baldin SL, Bernardo HT, de Farias AC, Rico EP, Wanderley AG. Gallic acid reverses neurochemical changes induced by prolonged ethanol exposure in the zebrafish brain. Neurosci. 2021;455:251–62.
Salehi A, Rabiei Z, Setorki M. Effect of gallic acid on chronic restraint stress-induced anxiety and memory loss in male BALB/c mice. Iran J Basic Med Sci. 2018;21(12):1232.
Gao J, Hu J, Hu D, Yang X. A role of gallic acid in oxidative damage diseases: a comprehensive review. Nat Prod Commun. 2019;14(8):1934578X19874174.
Samad N, Jabeen S, Imran I, Zulfiqar I, Bilal K. Protective effect of gallic acid against arsenic-induced anxiety-/depression-like behaviors and memory impairment in male rats. Metab Brain Dis. 2019;34:1091–102.
Rico EP, Rosemberg DB, Seibt KJ, Capiotti KM, Da Silva RS, Bonan CD. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol Teratol. 2011;33(6):608–17.
Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol. 2016;17(1):1–20.
Akintunde JK, Irondi AE, Ajani EO, Olayemi TV. Neuroprotective effect of dietary black seed flour on key enzymes linked with neuronal signaling molecules in rats exposed to mixture of environmental metals. J Food Biochem. 2018;42(5):e12573.
Mattson MP. Addendum: Pathways towards and away from Alzheimer’s disease. Nature. 2004;431(7004):107.
Chtourou Y, Slima AB, Gdoura R, Fetoui H. Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat hippocampus. Neurochem Res. 2015;40(8):1563–75.
Damodaran T, Müller CP, Hassan Z. Chronic cerebral hypoperfusion-induced memory impairment and hippocampal long-term potentiation deficits are improved by cholinergic stimulation in rats. Pharmacol Rep. 2019;71(3):443–8.
Olasehinde TA, Olaniran AO. Neurotoxicity of anthracene and benz[a]anthracene involves oxidative stress-induced neuronal damage, cholinergic dysfunction and disruption of monoaminergic and purinergic enzymes. Toxicol Res. 2022;38(3):365–77.
Driver AS, Kodavanti PR, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol. 2000;22(2):175–81.
Stepanova A, Konrad C, Manfredi G, Springett R, Ten V, Galkin A. The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem. 2019;148(6):731–45.
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32(1):19–29.
Bagatini MD, Dos Santos AA, Cardoso AM, Mânica A, Reschke CR, Carvalho FB. The impact of purinergic system enzymes on noncommunicable, neurological, and degenerative diseases. J Immunol Res. 2018;2018:4892473.
Agostinho P, Madeira D, Dias L, Simões AP, Cunha RA, Canas PM. Purinergic signaling orchestrating neuron-glia communication. Pharmacol Res. 2020;162:105253.
Reichert KP, Castro MF, Assmann CE, Bottari NB, Miron VV, Cardoso A, Stefanello N, Morsch VM, Schetinger MR. Diabetes and hypertension: pivotal involvement of purinergic signaling. Biomed Pharmacother. 2021;137:111273.
Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8):959–69.
Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76(1):51–69.
da Rosa G, Alba DF, Silva AD, Miron VV, Morsch VM, Boiago MM, Stefani LM, Baldissera MM, Lopes MT, Mendes RE, da Silva AS. Impacts of Escherichia coli infection in young breeder chicks on the animal behavior and cerebral activity of purinergic and cholinergic enzymes involved in the regulation of molecules with neurotransmitter and neuromodulator function. Microb Pathog. 2020;138:103787.
Author information
Authors and Affiliations
Contributions
Project design: AAA, AOA, and GO. Experimental: AAA and AOA. Data analysis: AAA. Writing—original draft: AAA and AOA. Writing—review and editing: AAA, AOA, and GO.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Handling of experimental animals was in accordance with international guidelines, and the procedure was approved by Ethical Committee of the Centre for Research and Development, Federal University of Technology, Akure, Nigeria (Approval number: FUTA/ETH/21/10).
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adebayo, A.A., Ademosun, A.O. & Oboh, G. Gallic acid abrogates cadmium-induced neurochemical changes and cognitive deficits in Wistar rat. Nutrire 48, 30 (2023). https://doi.org/10.1186/s41110-023-00216-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-023-00216-9