Abstract
The treatment of diabetes involves the use of herbal plants, attracting interest in their cost-effectiveness and efficacy. An aqueous extract of Persea americana seeds (AEPAS) was explored in this study as a possible therapeutic agent in rats with diabetes mellitus. The induction of diabetes in the rats was achieved by injecting 65 mg/kg body weight (BWt) of alloxan along with 5% glucose. This study was conducted using thirty-six (36) male Wistar rats. The animals were divided into 6 equal groups, (n = 6) and treated for 14 days. In vitro assays for total flavonoid, phenols, FRAP, DPPH, NO, α-amylase, and α-glucosidase, were performed. Biochemical indices fasting blood sugar (FBS), BWt, serum insulin, liver hexokinase, G6P, FBP, liver glycogen, IL-6, TNF-α, and NF-ĸB in the serum, were investigated as well as the mRNA expressions of PCNA, Bcl2, PI3K/Akt in the liver and pancreas. The in vitro analyses showed the potency of AEPAS against free radicals and its enzyme inhibitory potential as compared with the positive controls. AEPAS showed a marked decrease in alloxan-induced increases in FBG, TG, LDL-c, G6P, F-1, 6-BP, MDA, IL-6, TNF-α, and NF-ĸB and increased alloxan-induced decreases in liver glycogen, hexokinase, and HDL-c. The diabetic control group exhibited pancreatic dysfunction as evidenced by a reduction in serum insulin, HOMA-β, expressions of PI3K/AKT, Bcl-2, and PCNA combined with an elevation in HOMA-IR. The HPLC revealed luteolin and myricetin to be the phytochemicals that were present in the highest concentration in AEPAS. The outcome of this research showed that the administration of AEPAS can promote the activation of the PI3K/AkT pathway and the inhibition of β-cell death, which may be the primary mechanism by which AEPAS promotes insulin sensitivity and regulates glycolipid metabolism.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is among the top 10 reasons for premature death, accounting for over 1,000,000 deaths1 worldwide. Though there are two broad categories of diabetes, type-1 diabetes mellitus (T1DM) and type-2 diabetes mellitus (T2DM) which is the most widespread type. T2DM is a public health challenge and has contributed to human morbidity and premature mortality2. The prevalence of T2DM is 462 million in 2017 with a worldwide incidence rate of 6059 cases per 100,000 people and is projected to increase to 7079 cases per 100,000 people by 20303. The incidence of T1DM was 15 per 100,000 people and the prevalence was 9.5% (95% CI 0.07 to 0.12) in the world, which was statistically significant4.
Diabetes mellitus is a metabolic disorder linked to aberrant glucose concentration in the plasma and leads to insulin deficiency and/or impaired insulin activity. Some factors that affect insulin expression and action are body mass index (BMI), physical inactivity, heavy alcohol consumption, genetic and epigenetics, predisposition, and tobacco smoking4.
The characteristic feature of T1DM is often associated with autoimmune destruction of the pancreatic ß-cell, causing total insulin deficiency. The classic symptoms include weight loss, polyuria, polydipsia, polyphagia, and ketoacidosis. Pancreatic ß-cells are essential for maintaining a balance in blood glucose levels by synthesizing and secreting insulin5. Prolonged hyperglycemia results in the apoptotic death of pancreatic ß-cells, thus reducing the pancreatic ß-cell volume and stimulating aberrant insulin release and glucose uptake6. Hyperglycemia also stimulates the heightened generation of reactive species via NADPH oxidase activity, which potentiates proinflammatory biomarkers, such as IL-1β, IL-6, and TNF-α7.
Diabetes and its complications, like other diseases, have been linked to free radical generation, with glucose autoxidation being a major source of free radicals in chronic hyperglycemia7. Although free radical production is necessary for normal cellular homeostasis and the body's response to pathogens, many diabetes complications are caused by excessive free radical generation and oxidative stress6. The oxidative stress and inflammation caused by persistent hyperglycemia are major contributors to these diabetes complications8.
PI3K is a kinase that is involved in regulating both the uptake and utilization of cellular glucose. Based on experimental findings, it is now clear that the release and regulation of insulin by pancreatic cells takes place via a PI3K/AKT mediated pathway9,10. In addition to stimulating pancreatic β-cell survival by inhibiting FoxO1, it is implicated in lipotoxicity in pancreatic β-cells. Many studies have shown that the overexpression and activation of PI3K/AKT in β-cells stimulate insulin secretion while the overexpression of inactive mutated forms of this kinase in β-cells and a subsequent reduction in PI3k/AKT activity leads to a lack of insulin secretion10,11,12.
Persea americana Mill. (avocado) seed extract pulp, and leaves are rich in phytochemicals, vitamins, micronutrients, and antioxidants13,14. Research has identified Persea americana as a potent hypoglycemic agent14,15,16. However, the mechanism by which P. americana works and its hypoglycemic activity have not been explained. Therefore, our study examined the role of P. americana Mill. seeds in attenuating alloxan-induced diabetes by suppressing oxidative stress, inflammation, and β-cell apoptotic death, and by upregulating glucose uptake by stimulating the PI3K/AKT signaling pathway.
Results
HPLC–UV analysis of AEPAS
The outcomes of the HPLC analysis of AEPAS at 254 nm (Fig. S1), as shown by the chromatograms obtained at different retention times, showed the presence of several constituents, which are ascorbic acid, myricetin, luteolin, and gallic acid (Table 1).
In vitro antioxidant activity of AEPAS
As shown in Fig. 1, we compared the phenol and flavonoid contents of the AEPAS with standard gallic acid and quercetin. The graph shows the total phenolic content in the sample and the standard, which increases with concentration. The total flavonoid content was concentration dependent as the results showed an increase in flavonoid content with increased concentration. Figure 2 shows an increase in the total antioxidant capacity of AEPAS at a higher concentration. The total antioxidant ability of the extract was greater than α-tocopherol, which was used as the standard. The trends observed in the samples and standards were similar, as both showed increased antioxidant capacity at higher concentrations. Increasing the concentration increased the nitric oxide scavenging capability of AEPAS and gallic acid. We illustrate this in Fig. 2, which shows that the DPPH scavenging ability of AEPAS increases at higher concentrations with a concentration-dependent increase. We observed a similar trend in DPPH activity for standard vitamin C, as shown in Fig. 2. Figure 2 also shows that the reducing power of AEPAS depends on the concentration in Fig. 2.
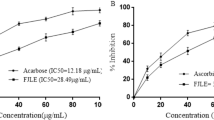
α-glucosidase and α-amylase inhibitory activities of AEPAS
As shown in Fig. 3, acarbose, as a reference drug, had a maximal inhibitory activity of α-glucosidase compared to aqueous seed extract. AEPAS showed its maximal α-glucosidase inhibitory activity at 56.41% and acarbose at 76.41%. In addition, Fig. 3 shows the percent α-amylase inhibitory activities of AEPAS and acarbose. The α-amylase inhibitory property exhibited in the aqueous extract was 21.42% compared to 76.41% for acarbose.
Fasting blood glucose (FBG) level of diabetic rats administered AEPAS
The FBG level of the male rats was elevated 72 h after induction of alloxan to values above 250 mg/dl, which were maintained in the untreated group for the duration of the experiment. A decline in FBG levels was observed after the administration of AEPAS at 26.7 mg/kg, 53.3 mg/kg, and 106.6 mg/kg BWt (Table 2). The reduction in the FBG level in the animal groups treated with 53.3 mg/kg and 106.6 mg/kg BWt yielded values in alignment with the nondiabetic group (Table 2). We found the most profound effect at 106.6 mg/kg BWt.
Body weight of diabetic rats administered AEPAS
We witnessed a notable decrease in the body weight in the animals, apparently resulting from the administration of alloxan (Table 3), as evidenced by the weight gain in the normal control group. Treatment with AEPAS at 26.7 mg/kg, 53.3 mg/kg, and 106.6 mg/kg BWt resulted in slight increases in the BWt of the rats (Table 3) with the increases being dose-dependent.
Serum insulin levels, HOMA-IR and HOMA- β levels of diabetic rats administered AEPAS
From the results presented in Table 4, the serum insulin and HOMA-β levels of the diabetic untreated group showed a remarkable decrease whereas the HOMA-IR levels increased. Administration of AEPAS at 26.7 mg/kg, 53.3 mg/kg, and 106.6 mg/kg resulted in elevated serum insulin and HOMA-β levels with a noteworthy decrease in the HOMA-IR levels.
Antioxidant markers in experimental DM
The activities of glutathione peroxidase (GPx), glutathione-S-transferase (GST), reduced glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) in the liver were in contrast to the untreated diabetic rats when either AEPAS or metformin was administered to diabetic rats. Lipid peroxidation (MDA) levels decreased after administration of either metformin or AEPAS, in contrast to the elevated levels in the untreated diabetic rats, and the results were dose-dependent (Table 5). The activities of GPx, GST, GSH, CAT, and SOD in the pancreas were elevated and the MDA levels decreased after administration of AEPAS and metformin compared with the diabetic untreated rats (Table 6).
Serum lipid parameters in experimental DM administered AEPAS
Administration of alloxan led to an increased concentrations of VLDL-c, LDL-c, TG, CRI, AI, and total cholesterol with a reduction in HDL-c levels (Table 7). Administration of the various dosages of AEPAS at 26.7 mg/kg, 53.3 mg/kg, and 106.6 mg/kg resulted in a decrease in triglycerides, LDL-c, total cholesterol, CRI, VLDL-c, and AI levels in contrast to the diabetes-induced rats. The doses of the extract elevated the serum HDL-c levels when compared to the diabetic rats. Metformin treatment resulted in a significant decrease in triglycerides, LDL-c, total cholesterol, CRI, VLDL-c, and AI levels with a corresponding improvement in HDL-c levels.
Hepatic glycogen and carbohydrate metabolizing enzymes in diabetic rats administered AEPAS
The levels of hepatic glycogen and the glycolytic enzyme, hexokinase, were diminished in the diabetic rats, and we observed a remarkable increase of these in the treatment groups after administration of either metformin or AEPAS (Table 8). We found that the activities of the gluconeogenesis enzymes G6Pase and F-1,6-BPase increased in the diabetic control group, but administration of either AEPAS or metformin yielded a notable decrease in the treatment groups (Table 8).
Pro- and anti-inflammatory markers of diabetic rats administered AEPAS
Administration of alloxan increased the levels of IL-6, TNF-α, and NF-κB (Fig. 4) in the plasma of the rats (p < 0.05) compared with those of the control rats. AEPAS reduced the levels of IL-6, TNF-α, and NF-κB (p < 0.05) compared to the diabetic rats, as did treatment with metformin. AEPAS at 26.7, 53.3, and 106.6 mg/kg BWt showed the reversal of the alloxan treatment-related rises in the concentrations of IL6, TNF-α, and NF-κB (Fig. 4).
Plasma pro- and anti-inflammatory markers of diabetic rats administered AEPAS, (A) interleukin-6, (B) tumor necrosis factor-α, and (C) nuclear factor-kappa B of alloxan-induced diabetic rats orally administered AEPAS. Data are expressed as mean ± SEM (n = 6); AEPAS: aqueous extract of Persea americana seeds; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; NF-κB: Nuclear factor-kappa B; #: significantly different from normal control (P < .05); *, **,*** is significantly different from diabetic control at P < 0.05, < 0.01, < 0.001).
Gene expressions of PI3K, AKT, and apoptotic markers of diabetic rats administered AEPAS
The PI3K, AKT, Bcl-2, and PCNA mRNA expression levels in the liver and pancreas are shown in Fig. 5. The the mRNA expression levels of PI3K, AKT, Bcl-2, and PCNA were down-regulated in the liver and the pancreas of the diabetic rats (Fig. 5). Treatment with 26.7 or 53.3 mg/kg AEPAS and metformin raised the mRNA expression levels of PI3K and AKT in the liver, while AEPAS at all doses increased the expression of Bcl-2 and PCNA in the liver. In addition, AEPAS at all doses and metformin caused an elevation in the mRNA expression of PI3K, AKT, and Bcl-2 in the pancreas. In contrast, the PCNA mRNA expression at 26.7 and 53.3 mg/kg increased in the diabetic rats but decreased at 106.6 mg/kg AEPAS in the pancreas.
Effect of AEPAS on PI3K, AKT, Bcl2, and PCNA in the liver and pancreas of diabetic rats. AEPAS: aqueous extract of P. americana seeds; PCNA: proliferating cell nuclear antigen; CT: control group; DC: diabetic control; DM: diabetic + metformin group; PA-1: P. americana treated group (26.7 mg/kg); PA-2: P. americana treated group (53.3 mg/kg) PA-3: P. americana treated group (106.6 mg/kg).
Histological study of pancreatic tissues
Alloxan induction resulted in the partial destruction of the β-cells of the pancreatic islets when compared to control rats (Fig. 6). Treatment with AEPAS, on the other hand, improves and restores the damaged pancreatic islets cells at all doses.
Cross section of the pancreas of rats after administration of aqueous extract of P. americana seeds. (A) NC, Normal control; (B) DBC, Diabetic Control; (C) DMF, Diabetic Metformin; (D) DPa26.7: Diabetic P. americana aqueous seed extract (26.7 mg/kg body weight); (E) DPa53.3: Diabetic P. americana aqueous seed extract (53.3 mg/kg body weight); (F) DPa106.6: Diabetic P. americana aqueous seed extract (106.6 mg/kg body weight).
Discussion
Researchers have related the antidiabetic properties of several medicinal plants to bioactive compounds such as phenolics, flavonoids, and tannins17. Over the years, scientists have explored these chemical compounds in managing several diseases by folklore medicine18,19,20. The HPLC of AEPAS confirmed the presence of ascorbic acid, myricetin, luteolin, and gallic acid. Ascorbic acid is available as a natural antioxidant in some systems. Advanced glycation end products, glycosylation of proteins, the polyol pathway, and auto-oxidation of glucose are involved in the pathogenesis of T1DM and T2DM. Thus, one important defense against such impairment is antioxidant compounds such as ascorbic acid21.
Myricetin, a flavonoid compound, was documented to have antioxidant and antidiabetic activities. Myricetin facilitated the metabolic action of insulin by stimulating phosphatidylinositol 3-kinase (PI3K) and its effectors22.
Luteolin is an active flavonoid with an extensive array of pharmacological properties. The well-known pharmacological properties of luteolin, for example, its antioxidant, anti-inflammatory, anti-apoptotic, and antidiabetic properties, have been demonstrated23.
The antioxidant property and inhibitory activities of gallic acid on marker enzymes (α-glucosidase and α-amylase) linked to diabetes mellitus have been shown by Mackensie et al.24, and improvement in IR sensitivity by gallic acid may account for its anti-diabetic property25. All the activities of ascorbic acid, myricetin, luteolin, and gallic acid summarized above could have accounted for the antioxidant and antidiabetic properties we observed for AEPAS.
Recently, phenolic compounds have attracted considerable attention for their promising use as novel nutraceuticals or biological products because of their notable antiradical and anti-inflammatory properties, which may be concentration dependent26. Flavonoids are a major group of secondary metabolites, and many experiments have reported their biological properties27. This study showed that the total phenolic and flavonoid content of AEPAS operated in a concentration-dependent manner.
Free radicals have been linked to diseases such as T2DM28. Researchers documented that hyperglycemia produces free radicals that turn on the free radical/antioxidant defense system, producing oxidative stress. We can evaluate the antioxidant activities of therapeutic plants via their total antioxidant capacity and their reducing and radical scavenging properties29. Recent studies reported that strong antioxidants present in therapeutic foods and plants can be useful in neutralizing the effects of stress in diseases such as type-2 diabetes30. The efficiency of AEPAS as an antioxidant could be due to phenolics and flavonoids, a conclusion that is further supported by the ability of AEPAS to reduce ferric ions and to neutralize NO and DPPH radicals. From this study, AEPAS revealed a high total antioxidant capacity, reducing capacity, and scavenging power. An overabundance of nitric oxide can lead to tissue injury and Type-2 diabetes-linked cardiovascular problems31. Hence, AEPAS could inhibit nitric oxide radicals in a concentration-dependent manner. This could be because of the compounds, including ascorbic acid, myricetin, luteolin, and gallic acid, present in AEPAS, which have been reported to have these properties.
The treatment of hyperglycemia is the essential goal for managing diabetes mellitus. One of the most significant beneficial methods is reducing postprandial hyperglycemia by hindering carbohydrate absorption and digestion32. Anti-diabetic agents function by inhibiting α-amylase and α-glucosidase enzymes relevant to T2DM33. These enzymes perform a key role in the breakdown of nutritional carbohydrates to glucose33. The inhibitory activity of the AEPAS on α-amylase and α-glucosidase indicates its potential as an antidiabetic treatment. The inhibition of these enzymes by AEPAS clarifies its capability to delay the breakdown of carbohydrates, hence controlling the FBG level. We can credit these activities to the metabolites, phenolics, and flavonoids that have been described as components of herbal extracts with anti-diabetic properties34. It is important to note that the α-amylase activity of AEPAS compared with that of acarbose, whose mechanism of action involves inhibiting these enzymes.
Alloxan-induced diabetes is a valuable experimental model for examining the antidiabetic properties of many agents35. The mechanism by which alloxan induces diabetes is that it selectively inhibits glucose-induced insulin production via a specific hexokinase inhibition that triggers a condition similar to T2DM via its capacity to induce ROS, resulting in pancreatic β-cell toxicity36. The reference antidiabetic agent, metformin, acts in many ways, including decreasing glucose generation, improving lipid oxidation in liver cells, and/or increasing the uptake of glucose36. The increased FGB level observed after 72 h of alloxan administration supports the idea that glucose-induced insulin production is inhibited through specific hexokinase inhibition and selective toxicity on the β-cells36. The decreased level of serum FBG in both the AEPAS and metformin-treated groups compared with the diabetic group supports the view that the therapeutic properties displayed by AEPAS could result from phenolics components such as gallic acid and myricetin. The reduction in serum glucose level by the AEPAS could be due to the increased uptake of glucose evidenced by the improved glycolytic pathway in this study.
A severe loss in bodyweight typifies alloxan-induced diabetes37. The difference in energy consumption and usage leads to an alteration in body weight37. The decrease in body weight in diabetic rats might be the result of a decrease in glucose metabolism, elevated metabolism of fats, or the structural breakdown of proteins that provide an alternate source of energy37. The increased body weight of the diabetic rats treated with AEPAS at the highest dose could have resulted from greater glycemic control through improved insulin secretion. This antidiabetic property indicates that AEPAS may promote insulin production from the residual β-cells or restored β-cells which could activate the hormones involved in fat storage.
Insulin resistance and pancreatic β-cell dysfunction typifies diabetes mellitus38. The quantitative assessment of insulin resistance (HOMA-IR) and insulin production/β-cell function (HOMA-β) are vital keys for measuring IR and assessing β-cell function38. Improved insulin production and insulin sensitivity in response to AEPAS showed its ability to improve HOMA-IR and HOMA-β. It would be reasonable to credit this effect to the flavonoids in AEPAS since they have been shown to promote the restoration of pancreatic β-cells and increase insulin release in diabetes-induced rats38. This corresponds to the findings of a previous study from our laboratory.
Enzymatic antioxidants (CAT, SOD, GPx, GSH, and GST) and non-enzymatic antioxidants (MDA) perform a crucial function in maintaining the biological levels of oxygen and hydrogen peroxide via improving the dismutation of O2 radicals and destroying organic peroxides produced from alloxan exposure39. This current study showed that alloxan-induced diabetes unbalanced the activity of liver marker enzymes. The observed reduction in the activities of CAT, SOD, GPx, and GST and the levels of GSH in the liver and pancreas of diabetic rats may be due to the chemical reduction of alloxan to dialuric acid to produce redox intermediates and of oxidized glutathione (GSSG) to produce radicals, such as the OH radical that is the main toxic ROS species36. This may account for the inadequacy of the defense system in alleviating ROS facilitated injury40. The improved activities of these enzymes by AEPAS appears to have reduced the difference between the production of ROS and enzymatic antioxidant activities in the diabetic rats. Also, the formation of ROS is prevented by the destruction of radicals by these antioxidant enzymes. Thus, the ability of AEPAS to attenuate the changed antioxidant enzymes in alloxan-induced diabetic rats indicates its radical scavenging property. This may be due to the phenolics and flavonoids in AEPAS, which have been identified as having radical scavenging effects27.
Dyslipidemia, increments in the concentrations of TC, TG, and alterations in lipoprotein components, is a recognized challenge in diabetic patients41,42. Also, the high concentrations of LDL-c and AI induced by alloxan imply a propensity for cardiovascular issues42. The increased levels of TC in diabetic animals are due to the inactivation of lipoprotein lipase41,42. The ability of AEPAS to restore the deranged metabolic pathways could be the result of lipase inhibition via insulin and a subsequent reduction in the rate of lipid degradation, biotransformation of free fatty acids to phospholipids. This effect by AEPAS might result from the presence of flavonoids, which have been reported to reduce lipid metabolism42.
Glycogen concentrations in tissues such as the liver are a direct indication of insulin action in that insulin improves internal glycogen deposition by inhibiting glycogen phosphorylase43. Depletion of glycogen by alloxan administration could result from a decrease in the activity of hexokinase. The restoration of hepatic glycogen after the administration of AEPAS may have resulted from its ability to improve insulin release from the pancreas43. However, this could also have been because of polyphenols, which have been reported to have insulin mimetic activity thus, giving rise to direct peripheral glucose uptake43.
The liver is a significant organ that performs a vital function in the glycolytic and gluconeogenic pathways. Hexokinase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase are key enzymes in glucose metabolism44. The decrease in hexokinase activity in diabetic animals could be because of reduced glycolysis and diminished use of glucose for energy creation. Increased activity resulting from AEPAS appears to have enhanced the use of glucose for energy creation, which suggests a higher glucose uptake from the blood by hepatocytes and improved glycolysis.
The activity of these enzymes (F-1,6-BPase and G-6-Pase may be elevated in diabetes45. The increase in F-1,6-BPase and G-6-Pase in the hepatic tissue of diabetic rats may be related to insulin inadequacy and overproduction of glucose46. In addition, the decrease in F-1,6-BPase and G-6-Pase after AEPAS treatment could have resulted from inhibiting gluconeogenesis through improved insulin production, modulating the activity of F-1,6-BPase and G-6-Pase via the control of cyclic adenosine monophosphate (cAMP) or inhibition of glycolysis47. The ability of AEPAS to modulate F-1,6-BPase and G-6-Pase could be the result of the action of flavonoids and phenolics, which play a vital role in reversing F-1,6-BPase and G-6-Pase to near normal through enhanced insulin secretion by their antioxidant potential. This further supported the antihyperglycemic activity of the AEPAS.
The PI3K/AKT signaling pathway is linked to the glucose metabolism essential for insulin stimulated glucose intake in the liver, as earlier documented by Nandipati et al.48, and Yu et al.49 Akt activation stimulated the cell’s existence by phosphorylation50. It facilitated the metabolic action of insulin through the activation of PI3K and its effectors, the protein kinase B (PKB/Akt) kinases. In addition, the AMPK signaling pathway may facilitate the impact of the insulin-independent response to glucose uptake. AEPAS could protect against alloxan-induced damage through an anti-apoptotic effect by increasing the mRNA expression of the phosphorylation of Akt50. AEPAS reversed the reduced mRNA expression of PI3K and AKT levels in diabetic rats (Fig. 7).
Proposed mechanism of action of Persea americana seeds in diabetic rats on improving insulin binding and increasing glucose metabolism. P. americana, increases the PI3K/Akt, PCNA, and Bcl2 expression in the insulin signaling pathways. This leads to an increase in insulin sensitivity and a reduction in blood glucose.
The antiapoptotic Bcl-2 is a major molecule involved in apoptosis. Alloxan-induced diabetic rats had reduced Bcl-2 expression in the liver and pancreas tissues while treatment with AEPAS altered the balance of the antiapoptotic (Bcl-2) molecules and prevented cell death of the hepatic and pancreatic cells at all doses, as did metformin. The findings from this study (elevated MDA level and reduced SOD activity in the liver and pancreatic tissues of diabetic rats) agree with a study describing the elevated generation of radicals resulting in cell death of hepatic and pancreatic cells51. In this case, the apoptotic pathway requires additional examination to determine whether caspase(s) and cytochrome c are involved, as documented in other research experiments52.
As a valuable proliferation marker, proliferating cell nuclear antigen (PCNA) expression performs exclusive functions at the start of cell propagation by facilitating DNA polymerase. PCNA also performs key functions in the eukaryotic cell cycle in addition to stimulating the development of antibodies towards foreign compounds53. In this study, the mRNA expression of PCNA was up-regulated in the hepatocytes and pancreatic tissues of the normal rats and downregulated in the diabetic rats. AEPAS improved the PCNA expression in the liver and pancreatic tissues of diabetic rats.
In conclusion, AEPAS attenuates insulin resistance in alloxan-induced diabetic rats. The potential molecular mechanisms of AEPAS are an increase in glucose uptake, increased hexokinase activity, increased insulin levels, and a decrease in pancreatic β-cells apoptosis via activation of the PI3K/Akt signaling pathway in diabetic rats (Fig. 7). AEPAS could be an excellent source of antidiabetic agents as it controls the hyperglycemic index and other associated biochemical indices.
Materials and methods
Chemicals
Alloxan was purchased from Sigma-Aldrich, Steinheim, Germany. Methanol, and Folin–Ciocalteu’s reagents were both acquired from Merck (Darmstadt, Germany); α-amylase, diphenylamine, acarbose, and α-glucosidase were all products of Sigma-Aldrich, Steinheim, Germany. All chemical agents, as well as standards, were analytical grade, unless otherwise noted.
Plant material
The seeds of P. americana were collected from a local farm settlement in Ado-Ekiti metropolis, Ekiti State, after obtaining permission from the farm owner to collect the seeds of P. americana on October 10, 2020. Mr. Odewo at the Forestry Research Institute of Nigeria (FRIN) identified the plant and provided FHI 113162 as the voucher number. The link http://www.theplantlist.org was used to check the name of the plant. Studies complied with local and national ethical guidelines/regulations on the usage of plants.
Preparation of aqueous extract of P. americana seeds (AEPAS)
Persea americana seeds were sliced into smaller parts and dried for 4 weeks at 25 °C, after which they were ground into powder using an electric blender (Kenwood, Model BL490, China). 35 g of dried seed was soaked in distilled water for 48 h to obtain the aqueous extracts17. We concentrated the aqueous extract obtained using a freeze-dryer (Modulyo Freeze Dryer, Edward, England) to obtain an 18.5 g yield.
High-performance liquid chromatography (HPLC–UV) analysis
We evaluated the HPLC analysis of AEPAS by a chromatographic system (N 2000, Korea) utilizing an Autosampler (YL 9150) with 100 μl fixed loop and a YL9120 UV–visible detector. We did the separation on an SGE Protocol PC18GP120 (250 mm × 4.6 mm, 5 μm) column at 25 °C. The mobile phase contailed methanol to water (70:30 v/v), and we utilized the isocratic mode; elution was achieved at a flow rate of 1 ml/min. The samples were run for 15 min and absorbance was read at 254 nm. The chromatographic data were assessed using the Autochro-2000 software.
Total phenolic content determination
The technique described by Singleton et al.54 was used to determine the phenolic content. 200 µl of AEPAS at varying concentrations of 15–240 µg/ml was added to test tubes containing 2 ml of NaHCO3. 200 µl of Folin-Ciocalteu (Folin-C) reagent was added two minutes later, after which the mixtures were well mixed and incubated in the water bath for 30 min at 50 °C. We read the absorbance at 760 nm. The standard gallic acid was prepared in the same way as the AEPAS stock solution. The phenolic content was determined using the same protocol as used for AEPAS with the standard being used in place of AEPAS.
Total flavonoid content determination
The aluminum chloride (AlCl3) procedure was used to determine the total flavonoid content of AEPAS55. The stock solutions of AEPAS were prepared at concentrations of 15–240 µg/ml and 1 ml of AEPAS was measured into clean tubes, after which 3 ml of distilled water and 0.3 ml of 5% NaNO2 were added. 0.3 ml of 10% AlCl3 and 2 ml of 1 M NaOH were added 5 min later and the volume of the solution in the test tube was diluted to 10 ml by adding distilled water. We read the absorbance at 510 nm. Quercetin was used as a reference and prepared by dissolving 4 mg in 1 ml of methanol. Various concentrations were prepared, and we assayed the flavonoid content using the same method outlined above for AEPAS.
Assessment of in vitro antioxidant capacity
Total antioxidant activity (TAC)
The protocol described by Prieto et al.56 was used to determine the TAC. 1 ml of the reagent (28 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulfuric acid) and 100 µl (0.1 ml) of AEPAS at various concentrations were placed in test tubes. The test tubes were then capped using foil paper and incubated in a water bath at 95 °C for about 90 min. The samples were then cooled, and we read the absorbance at 695 nm.
Assay of DPPH radical scavenging ability
We adopted the methodology described by Oboh and Rocha57 to determine the 2, 2- diphenyl-1-picrylhydrazyl scavenging effect of AEPAS. In brief, the extract (1 ml) at concentrations ranging from 15 to 240 µg/ml was added to 0.4 mM of DPPH solution prepared with methanol. Before reading, we kept the solution in the dark for 30 min, after which we read the absorbance of the mixture at 517 nm. This was done in triplicate. The reference used was ascorbic acid. The percentage of DPPH discoloration was then further calculated.
Assay of NO radical scavenging ability
The method described by Jagetia and Baliga58 estimated the capacity of nitric oxide to scavenge radicals in AEPAS. Varying concentrations (15–240 µg/ml) of AEPAS and the reference were prepared. 2.5 ml of 10 mM sodium nitroprusside (SNP) prepared in PBS (phosphate buffered saline) was added to 0.5 ml of varying concentrations of AEPAS and standard. The mixture was then further incubated for 150 min at 25 °C. A 0.5 ml aliquot was removed after the incubation period, and 0.5 ml of Griess reagent, which contains 1% (w/v) of sulphanilamide, 2% (v/v) of H3PO4, and 0.1% (w/v) of naphthylethylenediamine dihydrochloride, was added. We used 2 ml of sodium nitroprusside in PBS as the reference sample. The NO scavenging radical activities of AEPAS and gallic acid were both calculated and expressed as percentages.
Reducing ability
The reducing ability of AEPAS was assessed using Pulido et al.’s procedure59. We dissolved 2.5 ml of the sample (powdered) in about 2.5 ml of 200 mM sodium phosphate buffer of pH 6.6 and about 2.5 ml of 1% potassium ferricyanide. The mixtures were further incubated for 20 min at 50 °C, and then about 2.5 ml of 10% TCA was introduced to terminate the reaction. This was centrifuged for 10 min at 3000×g after which we added about 5 ml of the supernatant, an equivalent volume of water, and about 1 ml of 0.1% ferric chloride. The procedure followed above was also repeated, but in this case, it was for ascorbic acid, which was used as the reference sample and the reaction absorbance was read at 700 nm, followed by the calculation of the reducing ability which was expressed as an ascorbic acid equivalent.
α-Amylase inhibitory activity
The inhibitory activity of α-amylase in AEPAS was determined following Shai et al.’s protocol60. 250 µl of the sample at various concentrations ranging from 15 to 240 µg/ml was then incubated along with about 500 µl of 2 U m/l porcine pancreatic amylase in 100 mmol/l of phosphate buffer with a pH of 6.8 for 20 min at 37 °C. Then, 250 µl of starch (about 1%) dissolved in phosphate buffer of pH 6.8 (100 mmol/l) was added to the mixture and kept warm for 1 h at 37 °C. 1 ml dinitrosalicylic acid containing (3,5-dinitrosalicylic acid (1%), phenol (about 0.2%), Na2SO3 (0.05%) and sodium hydroxide (1%)) color reagent was further added; after which we heated it for 10 min at 100 °C. A cold water bath was then used to cool down the mixture to about 25 °C, then the absorbance of the resulting mixture was read on the spectrophotometer at 540 nm. The standard used for this study was acarbose. The result was calculated and expressed as a percentage.
α-Glucosidase inhibitory activity
The inhibitory activity of α-glucosidase by AEPAS was determined via a procedure provided by Nguelefack et al61. 1 mg of α-glucosidase was dissolved in a phosphate buffer (100 ml) at a pH of about 6.8 and containing 200 mg of bovine serum albumin (BSA). The mixture, which contained 10 µl of the sample at various ranges of concentration of about 15–240 µg/ml was remixed with 490 µl of phosphate buffer with a pH of about 6.8 and 250 µl of 5 mM p-nitrophenyl α-d-glucopyranoside. Pre-incubation for 15 min at 37 °C, after which there was an addition of 2000 µl Na2CO3 (200 mM) to stop the reaction. The α-glucosidase activity was estimated using a spectrophotometer to take the reading at 400 nm. The positive control used was acarbose of α-glucosidase inhibitor.
Experimental animals
36 males experimental Wistar rats (245.54 ± 10.52 g) were bought from the Animal Holding Unit of the Department of Biochemistry, University of Ilorin, in Ilorin, Kwara State. The experimental animals were kept in cages (temperature: 25 ± 27 °C; as well as a photoperiod time of 12 h natural light and 12 h darkness; relative humidity between 40–45%. They were fed with animal feed (Top Feeds, Beside First Bank Plc, Adebayo, Ado-Ekiti, Nigeria) and freely available tap water. The animals were acclimatized for 14 days before we initiated the experiment.
Dosage determination
Ethnobotanical survey and personal communications with traditional medicine practitioners revealed that around 200 ml of the juice extract is administered two times in a day for effective treatment of DM by a patient weighing about 70 kg. However, 18.46 g of AEPAS extract was derived after freeze-drying 200 ml of the juice. From this extrapolation, 53.3 mg/kg BWt was adopted as a dosage to determine the acclaimed anti-hyperglycemic potential of the extract.
Diabetes mellitus induction
Induction of DM in rats was employed via the method described Ojo et al.17 Male Wistar rats (36) weighing 245.54 ± 10.52 g were fasted without food and given only water for 12 h. The fasting blood sugar level of the rats was checked and recorded before the administration of alloxan. Afterward, 30 male Wistar rats were given a 65 mg/kg single injection (I.P.) of alloxan dissolved in normal saline (NaCl) and a 5% glucose solution to induce insulin resistance. We checked the fasting blood sugar level for each DM-induced animal after 72 h, to authenticate the induction of diabetes mellitus. Animals having an FBS level > 250 mg/dl were diabetic.
Animal groups and treatment with extract
Male Wistar rats (36) were selected and put into groups of six containing six rats each. The groupings were:
-
Group 1: Normal rats + distilled water
-
Group 2: Diabetic untreated rats
-
Group 3: Diabetic rats + 30 mg/kg BWt of metformin (oral gavage)
-
Group 4: Diabetic rats + 26.7 mg/kg BWt of AEPAS
-
Group 5: Diabetic rats + 53.3 mg/kg BWt of AEPAS
-
Group 6: Diabetic rats + 106.6 mg/kg BWt of AEPAS
The experiment lasted 14 days.
Ethical approval
All experimental rats that were used for this study were all handled in line with the rules and regulations set aside for animal management used in research as contained in the manual prepared for the care and use of animals in a laboratory. In addition, the Landmark University ethical committee approved this research and gave an approval number LUAC/2021/005A in compliance with ARRIVE guidelines.
Organ harvesting and analysis of samples
This research study lasted 14 days, after which we euthanized the rats using halothane. Then, the liver and pancreas were harvested and homogenized in a cold phosphate buffer, before being kept at a temperature of − 4°. The homogenized liver and pancreas were both centrifuged at 3000×g for 10 min to obtain a solution clear enough to evaluate some selected oxidative stress biomarkers. The blood was also collected and left for 1 h before centrifuging for 10 min at 3000×g to obtain a clear solution. We utilized this blood to determine the selected biochemical parameters.
Biochemical indices
We determined serum insulin concentration based on the method described by Ojo et al.17, which uses an ELISA kit from Sweden in a multiple plate ELISA reader (Winooski, Vermont, in the USA). We evaluated serum total cholesterol using the protocol from Fredrickson et al.62 Triglyceride was determined using the procedure described by Tietz63. Jacobs et al.’s64 protocol was used to evaluate the HDL-cholesterol. Both LDL and VLDL-cholesterol were evaluated using Friedewald et al.’s procedure65. The atherogenic index (AI) was determined using Liu et al.’s method66. Then the coronary artery index (CRI) was estimated utilizing Wilson and Islam’s process67.
Insulin resistance and β-cell function scores
Homeostasis model assessment of insulin resistance (HOMA-IR) was evaluated using Wilson and Islam’s method67. The homeostasis model assessment of the β-cell score (HOMA-β) was estimated using the equations utilized by Wilson and Islam67.
The conversion factors for units: insulin (1 U/l = 7.174 pmol/l) and blood glucose (1 mmol/l = 18 mg/dl).
Determination of biomarkers of oxidative stress
The supernatants of both the liver and pancreas were used to assay for reduced glutathione (GSH) level68, GPx69, catalase (CAT)37, and superoxide dismutase (SOD) activities70, and MDA level71.
Determination of the activities of glycolytic enzymes and glycogen level
The liver supernatant was used to analyze the activities of the glycolytic enzymes, including hexokinase72, glucose-6-phosphatase (G6Pase)73, and fructose 1,6-bisphosphatase74. We estimated the liver glycogen following the procedure described by Morales et al.75.
Determination of inflammatory biomarkers
TNF-α, IL-6, and NF-κB, were determined in the serum utilizing the procedure delineated in ELISA (Sigma Chemical Company Inc. (St. Louis, MO, USA)) kits.
Total RNA isolation
We removed total RNA from entire organs following a technique described by Omotuyi et al.76 The organs were homogenized in cool (4 °C) TRIzol reagent (Zymo Research, USA, Cat:R2050-1-50, Lot: ZRC186885). Absolute RNA was apportioned in chloroform solvent (BDH Analytical Chemicals, Poole, England Cat: 10076-6B) and centrifuged at 15,000 rpm for 15 min (Abbott Laboratories, Model: 3531, Lake Bluff, Illinois, United States). The RNA from the supernatant was precipitated using an equivalent amount of isopropanol (Burgoyne Urbidges & Co, India, Cat: 67-63-0). The RNA pellet was washed two times in 70% ethanol (70 ml absolute ethanol (BDH Analytical Chemicals, Poole, England Cat: 10107-7Y) in 30 ml of nuclease-free water (Inqaba Biotec, West Africa, Lot no: 0596C320, code: E476-500ML)). The pellets were air-dried for 5 min and solubilized in RNA buffer (1 mM sodium citrate, pH 6.4).
cDNA transformation
Before cDNA transformation, absolute RNA amount (concentration (µg/ml) = 40 * A260) and quality (≥ 1.8) were evaluated using the proportion of A260/A280 (A = absorbance) read using a spectrophotometer (Jen-way UV–VIS spectrophotometer model 6305, UK). DNA impurity detached from RNA was separated after DNAse I treatment (NEB, Cat: M0303S) as described by the manufacturer. A 2 µl solution containing 100 ng DNA-free RNA was changed to cDNA by employing the M-MuLV Reverse Transcriptase Kit (NEB, Cat: M0253S) in 20 µl final volume (2 µl, N9 random primer mix; 2 µl, 10X M-MuLV buffer; 1 µl, M-MuLV RT (200 U/µl); 2 µl, 10 mM dNTP; 0.2 µl, RNase Inhibitor (40 U/µl) and 10.8 µl nuclease-free water). The reaction continued at 25 °C O/N. Inactivation of M-MuLV Reverse transcriptase was achieved at 65 °C after 20 min.
PCR amplification and agarose gel electrophoresis
PCR intensification for the assessment of genes whose primers (Primer3 software) are recorded below were performed using the accompanying procedure: PCR enhancement was achieved in a 25 µl volume mixture containing 2 µl cDNA (10 ng), 2 µl primer (100 pmol) 12.5 µl Ready Mix Taq PCR master mix (One Taq Quick-Load 2x, master mix, NEB, Cat: M0486S) and 8.5 µl nuclease-free water. Early denaturation at 95 °C for 5 min was followed by 20 cycles of amplification (denaturation at 95 °C for 30 s, annealing for 30 s and amplified at 72 °C for 60 s), concluding with a final amplification at 72 °C for 10 min. In all the tests, we incorporated negative controls, in which the mixture had no cDNA. The amplicons were separated on 1.5% agarose gel (Cleaver Scientific Limited: Lot: 14170811) in Tris (RGT reagent, China, Lot: 20170605)-Borate (JHD chemicals, China, Lot 20141117)-EDTA buffer (pH 8.4).
PI3K mRNA Sequence (5′→3′)
-
Forward primerGGTGCTAAGGAGGAGCACTG
-
Reverse primerCCATGTGGTACAGGCCAGAG
AKT mRNA Sequence (5′→3′)
-
Forward primerAAGGACCCTACACAGAGGCT
-
Reverse primerAAGGTGGGCTCAGCTTCTTC
GAPDH mRNA Sequence (5′→3′)
-
Forward primerGCATCTTCTTGTGCAGTGCC
-
Reverse primerGAGAAGGCAGCCCTGGTAAC
Bcl-2 mRNA Sequence (5′→3′)
-
Forward primerGCGTCAACAGGGAGATGTCA
-
Reverse primerTTCCACAAAGGCATCCCAGC
PCNA mRNA Sequence (5′→3′)
-
Forward primerAGCAACTTGGAATCCCAGAACA
-
Reverse primerCACAGGAGATCACCACAGCA
Cyclophilin A mRNA Sequence (5′→3′)
-
Forward primerTGGAGAGCACCAAGACAGACA
-
Reverse primerTGCCGGAGTCGACAATGAT
Amplicon image processing
In-gel amplicon bands pictures were caught on camera analyzed using the Keynote platform according to Omotuyi et al.’s procedure76, and evaluated using image-J software.
Histology
After paraffin embedding, the fixed pancreatic tissues were stained with hematoxylin and eosin. A Leica slide scanner was used to view the slides (SCN 4000, Leica Biosystems, Germany).
Data analysis
We conducted the in vitro studies in triplicate. In the in vivo analysis, the data were all interpreted as mean ± SEM for six readings across the groups. We then subjected the data from this study to analysis by using one-way analyses of variance (ANOVA). Tukey’s post hoc comparison test was done using GraphPad Prism 9 version, and the significance level was set at P < 0.05.
Data availability
Data are available on request.
References
WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019 (World Health Organization, 2020).
Ramtahal, R. et al. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J. Epidemiol. Glob. Health 5, S35-43. https://doi.org/10.1016/j.jegh.2015.05.003 (2015).
Khan, M. A. B. et al. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 10, 107–111. https://doi.org/10.2991/jegh.k.191028.001 (2020).
Mobasseri, M. et al. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 10(2), 98–115 (2020).
Khamchan, A., Paseephol, T. & Hanchang, W. Protective effect of wax apple (Syzygium samarangense (Blume) Merr. & LM. Perry) against streptozotocin-induced pancreatic β-cell damage in diabetic rats. Biomed. Pharmacother 108, 634–645. https://doi.org/10.1016/j.biopha.2018.09.072 (2018).
Yaribeygi, H., Atkin, S. L. & Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell Physiol. 234(2), 1300–1312. https://doi.org/10.1002/jcp.27164 (2019).
Pickering, R. J. et al. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 7(4), 1–20. https://doi.org/10.1002/cti2.1016 (2018).
Rehman, K. & Akash, M. S. H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked?. J. Cell Biochem. 118, 3577–3585. https://doi.org/10.1002/jcb.26097 (2017).
Zhang, J. & Liu, F. Tissue-specific insulin signaling in the regulation of metabolism and aging. IUBMB Life 66, 485–495. https://doi.org/10.1002/iub.1293 (2014).
Huang, X., Liu, G., Guo, J. & Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14, 1483–1496. https://doi.org/10.7150/ijbs.27173 (2018).
Wang, H. W. et al. Glucagon-like peptide-1 and candesartan additively improve glucolipotoxicity in pancreatic beta-cells. Metabolism 60, 1081–1089. https://doi.org/10.1016/j.metabol.2010.11.004 (2011).
Wang, W. et al. Inhibition of Foxo1 mediates protective effects of ghrelin against lipotoxicity in MIN6 pancreatic beta-cells. Peptides 31, 307–314. https://doi.org/10.1016/j.peptides.2009.11.011 (2010).
Ding, H., Chin, Y. W., Kinghorn, A. D. & D’Ambrosio, S. M. Chemopreventive characteristics of avocado fruit. Semin. Cancer Biol. 17, 386–394. https://doi.org/10.1016/j.semcancer.2007.04.003 (2007).
Egwaoje, M. et al. Alterations of aqueous Persea americana seed extract on renal functions associated with diabetes mellitus in wistar rats. J. Adv. Med. Med. Res. 24, 1–8. https://doi.org/10.9734/jammr/2017/37956 (2017).
Samir, A. Z., Hesham, G.A.E.-R., Ahmed, M. B. & Khaled, S. E. Effect of Balanites aegyptiaca (heglig dates) and Persea americana (avocado fruit) on some hematological and biochemical parameters in streptozotocin induced diabetic male rats. Al-Azhar Bull. Sci. 29, 49–59. https://doi.org/10.21608/absb.2018.33818 (2018).
Ejiofor, C. C., Ezeagu, I. E. & Ayoola, M. Hypoglycaemic and biochemical effects of the aqueous and methanolic extract of Persea americana seeds on alloxan-induced albino rats. Eur. J. Med. Plants 26, 1–12. https://doi.org/10.9734/ejmp/2018/41587 (2018).
Ojo, O. A. et al. Ameliorative potential of Blighia sapida K.D. Koenig bark against pancreatic beta-cell dysfunction in alloxan-induced diabetic rats. J. Complement Integr. Med. https://doi.org/10.1515/jcim-2016-0145 (2017).
Atanasov, A. G. et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 33, 1582–1614. https://doi.org/10.1016/j.biotechadv.2015.08.001 (2015).
Ojo, O. A. et al. Spondias mombim L. (Anacardiaceae): Chemical fingerprints, inhibitory activities, and molecular docking on key enzymes relevant to erectile dysfunction and Alzheimer’s diseases. J. Food Biochem. 43, e12772. https://doi.org/10.1111/jfbc.12772 (2019).
Ojo, O. A. et al. Chromatographic fingerprint analysis, antioxidant properties, and inhibition of cholinergic enzymes (acetylcholinesterase and butyrylcholinesterase) of phenolic extracts from Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill bark. J. Basic Clin. Physiol. Pharmacol. 29, 217–224. https://doi.org/10.1515/jbcpp-2017-0063 (2018).
Dakhale, G. N., Chaudhari, H. V. & Shrivastava, M. Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharmacol. Sci. 2011, 195271. https://doi.org/10.1155/2011/195271 (2011).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806. https://doi.org/10.1038/414799a (2001).
Shimoi, K. et al. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 438, 220–224. https://doi.org/10.1016/s0014-5793(98)01304-0 (1998).
Hsu, C. L. & Yen, G. C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 98, 727–735. https://doi.org/10.1017/S000711450774686X (2007).
Huang, T. H. et al. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol. Appl. Pharmacol. 207, 160–169. https://doi.org/10.1016/j.taap.2004.12.009 (2005).
Mohamed, E. A. et al. Potent alpha-glucosidase and alpha-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement Altern. Med. 12, 176. https://doi.org/10.1186/1472-6882-12-176 (2012).
Ojo, O. A., Oloyede, O. I., Olarewaju, O. I. & Ojo, A. B. In vitro antioxidant activity and estimation of total phenolic content in ethyl acetate extract of Ocimum gratissimum. Pharmacologyonline 3, 37–44 (2013).
Francisqueti, F. V. et al. The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev. Assoc. Med. Bras. 1992(63), 85–91. https://doi.org/10.1590/1806-9282.63.01.85 (2017).
Seifu, D., F. Assefa & Abay, S. M. In Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy (ed Capasso, A.) 97–145 (Research Signpost, 2012).
Luisi, G., Stefanucci, A., Zengin, G., Dimmito, M. P. & Mollica, A. Anti-oxidant and tyrosinase inhibitory in vitro activity of amino acids and small peptides: New hints for the multifaceted treatment of neurologic and metabolic disfunctions. Antioxidants https://doi.org/10.3390/antiox8010007 (2018).
Adela, R. et al. Hyperglycaemia enhances nitric oxide production in diabetes: A study from South Indian patients. PLoS ONE 10, e0125270. https://doi.org/10.1371/journal.pone.0125270 (2015).
Talabi, J., Adeyemi, S., Awopetu, S., Ajiboye, B. O. & Ojo, O. A. Inhibitory effect of aqueous extracts of raw and roasted Sesamum indicum L. seeds on key enzymes linked to type-2 diabetes (α-amylase and α-glucosidase) and Alzheimer’s disease (acetylcholinesterase and butyrylcholinesterase). Potravinarstvo https://doi.org/10.5219/866 (2018).
Ojo, O. A. et al. Inhibitory effects of solvent-partitioned fractions of two Nigerian herbs (Spondias mombin Linn. and Mangifera indica L.) on alpha-amylase and alpha-glucosidase. Antioxidants https://doi.org/10.3390/antiox7060073 (2018).
Ibrahim, M. A., Koorbanally, N. A. & Islam, M. S. Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (alpha-glucosidase and alpha-amylase) by Khaya senegalensis. Acta Pharm. 64, 311–324. https://doi.org/10.2478/acph-2014-0025 (2014).
Ighodaro, O. M., Adeosun, A. M. & Akinloye, O. A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 53, 365–374. https://doi.org/10.1016/j.medici.2018.02.001 (2017).
Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51, 216–226. https://doi.org/10.1007/s00125-007-0886-7 (2008).
Yakubu, M. T., Uwazie, N. J. & Igunnu, A. Anti-diabetic activity of aqueous extract of Senna alata (Fabacea) flower in alloxan-induced diabetic male rats. Cameroon J. Biol. Biochem. Sci. 24, 7–17 (2016).
Wang, H. J. et al. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac. J. Clin. Nutr. 16(Suppl 1), 412–417 (2007).
Ajiboye, B. O. et al. Ameliorative potential of Sterculia tragacantha aqueous extract on renal gene expression and biochemical parameters in streptozotocin-induced diabetic rats. J. Pharm. Investig. 51, 103–113. https://doi.org/10.1007/s40005-020-00506-8 (2020).
Pari, L. & Latha, M. Antidiabetic effect of Scoparia dulcis: Effect on lipid peroxidation in streptozotocin diabetes. Gen. Physiol. Biophys. 24, 13–26 (2005).
Ojo, O. A., Ojo, A. B., Ajiboye, B. O., Imiere, O. D. & Oyinloye, B. E. Antihyperlipidemic activities and hematological properties of ethanol extract of Blighia Sapida Koenig bark in alloxan-induced diabetic rats. Serbian J. Exp. Clin. Res. 21, 11–17. https://doi.org/10.2478/sjecr-2018-0042 (2020).
Oloyede, O. B., Ajiboye, T. O., Abdussalam, A. F. & Adeleye, A. O. Blighia sapida leaves halt elevated blood glucose, dyslipidemia and oxidative stress in alloxan-induced diabetic rats. J. Ethnopharmacol. 157, 309–319. https://doi.org/10.1016/j.jep.2014.08.022 (2014).
Kalaivanan, K. & Pugalendi, K. V. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacognosy Res. 3, 67–71. https://doi.org/10.4103/0974-8490.79119 (2011).
Chakrabarti, S. et al. Advanced studies on the hypoglycemic effect of Caesalpinia bonducella F. in type 1 and 2 diabetes in Long Evans rats. J. Ethnopharmacol. 84, 41–46. https://doi.org/10.1016/s0378-8741(02)00262-3 (2003).
Vats, V., Yadav, S. P. & Grover, J. K. Effect of T. foenumgraecum on glycogen content of tissues and the key enzymes of carbohydrate metabolism. J. Ethnopharmacol. 85, 237–242. https://doi.org/10.1016/s0378-8741(03)00022-9 (2003).
Baquer, N. Z., Gupta, D. & Raju, J. Regulation of metabolic pathways in liver and kidney during experimental diabetes: Effects of antidiabetic compounds. Indian J. Clin. Biochem. 13, 63–80. https://doi.org/10.1007/BF02867866 (1998).
Ramalingam, S. et al. Hypoglycemic effect of black tea extract attenuates carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Int. J. Green Pharm. 13(2), 1–6 (2019).
Nandipati, K. C., Subramanian, S. & Agrawal, D. K. Protein kinases: Mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell Biochem. 426, 27–45. https://doi.org/10.1007/s11010-016-2878-8 (2017).
Yu, N. et al. Anti-diabetic effects of Jiang Tang Xiao Ke granule via PI3K/Akt signalling pathway in type 2 diabetes KKAy mice. PLoS ONE 12, e0168980. https://doi.org/10.1371/journal.pone.0168980 (2017).
Chen, L. et al. Hydroxysafflor yellow A protects against cerebral ischemia-reperfusion injury by anti-apoptotic effect through PI3K/Akt/GSK3beta pathway in rat. Neurochem. Res. 38, 2268–2275. https://doi.org/10.1007/s11064-013-1135-8 (2013).
Kanter, M., Aktas, C. & Erboga, M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol. Nutr. Food Res. 57, 1578–1585. https://doi.org/10.1002/mnfr.201200170 (2013).
Zhao, Y. et al. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol. Mech. Methods 23, 42–47. https://doi.org/10.3109/15376516.2012.731437 (2013).
Arikawe, A. P. et al. Comparison of streptozotocin-induced diabetic and insulin resistant effects on spermato-genesis with proliferating cell nuclear antigen (PCNA) immunostaining of adult rat testi. J. Exp. Clin. Med. 29, 209–214 (2012).
Singleton, V., Orthofer, R. & Lamuela-Raventós, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299, 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1 (1999).
Chang, C., Yang, M., Wen, H. & Chern, J. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 10, 178–182. https://doi.org/10.38212/2224-6614.2748 (2002).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. https://doi.org/10.1006/abio.1999.4019 (1999).
Oboh, G. & Rocha, J. B. T. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens). J. Food Biochem. 31, 456–473. https://doi.org/10.1111/j.1745-4514.2007.00123.x (2007).
Jagetia, G. C. & Baliga, M. S. Evaluation of the radioprotective action of geriforte in mice exposed to different doses of gamma-radiation. Am. J. Chin. Med. 32, 551–567. https://doi.org/10.1142/S0192415X04002193 (2004).
Pulido, R., Bravo, L. & Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 48, 3396–3402. https://doi.org/10.1021/jf9913458 (2000).
Shai, L. J. et al. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 76, 465–470. https://doi.org/10.1016/j.sajb.2010.03.002 (2010).
Nguelefack, T. B., Fofie, C. K., Nguelefack-Mbuyo, E. P. & Wuyt, A. K. Multimodal alpha-glucosidase and alpha-amylase inhibition and antioxidant effect of the aqueous and methanol extracts from the trunk bark of Ceiba pentandra. Biomed. Res. Int. 2020, 3063674. https://doi.org/10.1155/2020/3063674 (2020).
Fredrickson, D. S., Levy, R. I. & Lees, R. S. Fat transport in lipoproteins–an integrated approach to mechanisms and disorders. N. Engl. J. Med. 276, 34–42. https://doi.org/10.1056/NEJM196701052760107 (1967).
Tietz, N. W. Clinical Guide to LABORATORY Tests 3rd edn. (W.B. Saunders Company, 1994).
Jacobs, R. F., Sowell, M. K., Moss, M. M. & Fiser, D. H. Septic shock in children: Bacterial etiologies and temporal relationships. Pediatr. Infect. Dis. J. 9, 196–200. https://doi.org/10.1097/00006454-199003000-00010 (1990).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Liu, C. S., Lin, C. C. & Li, T. C. The relation of white blood cell count and atherogenic index ratio of LDL-cholesterol to HDL-cholesterol in Taiwan school children. Acta Paediatr. Taiwan 40, 319–324 (1999).
Wilson, R. D. & Islam, M. S. Fructose-fed streptozotocin-injected rat: An alternative model for type 2 diabetes. Pharmacol. Rep. 64, 129–139. https://doi.org/10.1016/s1734-1140(12)70739-9 (2012).
Livingstone, C. & Davis, J. Review: Targeting therapeutics against glutathione depletion in diabetes and its complications. Br. J. Diabetes Vasc. Dis. 7, 258–265. https://doi.org/10.1177/14746514070070060201 (2016).
Jurkovič, S., Osredkar, J. & Marc, J. Molecular impact of glutathione peroxidases in antioxidant processes. Biochem. Med. 18, 162–174 (2008).
Winterbourn, C. C. Superoxide as an intracellular radical sink. Free Radic. Biol. Med. 14, 85–90. https://doi.org/10.1016/0891-5849(93)90512-s (1993).
Varshney, R. & Kale, R. K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 58, 733–743. https://doi.org/10.1080/09553009014552121 (1990).
Zhang, Z. et al. Hypoglycemic and beta cell protective effects of andrographolide analogue for diabetes treatment. J. Transl. Med. 7, 62. https://doi.org/10.1186/1479-5876-7-62 (2009).
Erukainure, O. L., Mopuri, R., Oyebode, O. A., Koorbanally, N. A. & Islam, M. S. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed. Pharmacother. 96, 37–47. https://doi.org/10.1016/j.biopha.2017.09.106 (2017).
Gancedo, J. M. & Gancedo, C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch. Mikrobiol. 76, 132–138. https://doi.org/10.1007/BF00411787 (1971).
Morales, M. A., Jobaggy, A. J. & Terenzi, H. F. Mutations affecting accumulation of glycogen. Fungal Genet. Rep. https://doi.org/10.4148/1941-4765.1830 (1973).
Omotuyi, O. I. et al. Flavonoid-rich extract of Chromolaena odorata modulate circulating GLP-1 in Wistar rats: Computational evaluation of TGR5 involvement. 3 Biotech 8, 124. https://doi.org/10.1007/s13205-018-1138-x (2018).
Funding
The authors did not receive any funding from any organization.
Author information
Authors and Affiliations
Contributions
OAO, JCA, AIO, SG, and ABO designed and conceptualized the study, JCA, TCE, MI, DR, and NTA generated the data and analyzed the results, while OAO, TCA, BEO, and BOA wrote the manuscript draft and revised the manuscript for final submission. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojo, O.A., Amanze, J.C., Oni, A.I. et al. Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Sci Rep 12, 2919 (2022). https://doi.org/10.1038/s41598-022-07015-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07015-8
- Springer Nature Limited
This article is cited by
-
Phytomedicine approach for management of diabetes mellitus: an overview of scientifically confirmed medicinal plants with hypoglycaemic properties and their probable mechanism of action
Phytochemistry Reviews (2024)
-
A Dual Therapeutic Approach to Diabetes Mellitus via Bioactive Phytochemicals Found in a Poly Herbal Extract by Restoration of Favorable Gut Flora and Related Short-Chain Fatty Acids
Applied Biochemistry and Biotechnology (2024)
-
Polyphenolic contents, free radical scavenging properties, and enzyme inhibitory activities of Acacia nilotica (L.) delile seed and pod extracts
Vegetos (2023)
-
Investigation of antilipidemic efficacy of condensed tannins from three varieties of Sorghum bicolor seeds on high-fat diet-induced obese rats
Comparative Clinical Pathology (2023)
-
Antidiabetic properties of Dioscoreophyllum cumminsii (Stapf) Diels stem bark on streptozotocin-induced diabetic rats
Comparative Clinical Pathology (2023)