Abstract
Studies on 3T3-L1 cells and HepG2 hepatocytes have shown that phosphatidic acid phosphohydrolase1 (LPIN1) plays a key role in adipogenesis, acting as a co-activator of peroxisome proliferator-activated receptor gamma coactivator 1a (PGC-1a) to regulate fatty acid metabolism. However, the functional role and regulatory mechanism of LPIN1 gene in milk fat synthesis of buffalo are still unknown. In this study, overexpression of buffalo LPIN1 gene transfected with recombinant fusion expression vector significantly increased the expression of AGPAT6, DGAT1, DGAT2, GPAM and BTN1A1 genes involved in triglyceride (TAG) synthesis and secretion, as well as PPARG and SREBF1 genes regulating fatty acid metabolism in the buffalo mammary epithelial cells (BMECs), while the lentivirus-mediated knockdown of buffalo LPIN1 dramatically decreased the relative mRNA abundance of these genes. Correspondingly, total cellular TAG content in the BMECs increased significantly after LPIN1 overexpression, but decreased significantly after LPIN1 knockdown. In addition, the overexpression or knockdown of PPARG also enhanced or reduced the expression of LPIN1 and the transcriptional activity of its promoter. The core region of buffalo LPIN1 promoter spans from − 666 bp to + 42 bp, and two PPAR response elements (PPREs: PPRE1 and PPRE2) were identified in this region. Site mutagenesis analysis showed that PPARG directly regulated the transcription of buffalo LPIN1 by binding to the PPRE1 and PPRE2 on its core promoter. The results here reveal that the LPIN1 gene is involved in the milk fat synthesis of BMECs, and one of the important pathways is to participate in this process through direct transcriptional regulation of PPARG, which in turn significantly affects the content of TAG in BMECs.
Similar content being viewed by others
Introduction
Phosphatidic acid phosphohydrolase1 (LPIN1), as a member of the lipid protein family, was identified during positional cloning of the mutant gene underlying lipodystrophy in the fatty liver dystrophy (fld) mouse1. In mammals, there are three subtypes of LPIN: LPIN1, LPIN2 and LPIN3. They have the same structural characteristics, including at least one nuclear localization signal domain (NLS), one amino-terminal domain (N-LIP) and one carboxyl-terminal domain (C-LIP)2. The N-LIP and C-LIP are highly conserved in different mammals. All the members of LPIN not only play the role of phosphatidic acid phosphorylase (PAP), which are necessary for TAG synthesis, but also act as transcriptional co-activators to regulate the expression of genes related to the lipid synthesis3. Among the three LPINs, the PAP specific activity of LPIN1 was significantly higher than that of LPIN2 and LPIN3. In humans and mice, LPIN1 is highly expressed in adipose tissue, skeletal muscle, myocardium and testis, but low in liver, kidney, brain and other tissues4. In mouse liver and 3T3-L1 cells, LPIN1 deficiency can prevent normal lipid accumulation and induce expression of key adipogenic genes, including peroxisome proliferator-activated receptor (PPAR) and CCAAT enhancer-binding protein (C/EBP)5. In addition, studies have shown that LPIN1 gene can be induced to express in the late stage of 3T3-L1 preadipocyte differentiation6. After LPIN1 is interfered by siRNA, the 3T3-L1 cells cannot differentiate into mature adipocytes. On the contrary, the overexpression of LPIN1 gene can promote their differentiation and maturation6. These results reveal that LPIN1 plays an important role in adipocyte maturation and adipogenesis. Previous studies also showed that LPIN1 is the most highly expressed subtype of the LPIN family, and its expression level on the 60th day of lactation is 15 times higher than that on the 15th day before lactation, suggesting that LPIN1 may be involved in milk fat synthesis7. The LPIN1 is also a downstream gene of the PPARG signaling pathway in the mammary gland of dairy cows8. In yak, the expression level of LPIN1 during the peak lactation is higher than that during other lactation and dry period, further suggesting that this gene may be an important gene related to lactation process9.
Peroxisome proliferator-activated receptor gamma (PPARG) is a critical gene that regulates lipid metabolism, body immunity, insulin resistance and adipocyte differentiation10. In 3T3-L1 cells, overexpression of LPIN1 increases the expression of PPARG and the accumulation of lipid droplets, while knockdown of LPIN1 gene could inhibit the adipogenesis induced by PPARG agonists and also reduce phosphorylation levels of PPARG and mitogen-activated protein kinase (ERK1/2)11. The study by Kim et al12 also demonstrated that in the absence of ligand rosiglitazone, LPIN1 activated PPARG by releasing nuclear receptor corepressor 1 (NCOR1), retinoic acid and thyroid hormone receptor silencing mediator (SMRT). The physical interaction between LPIN1 and PPARG occurs in the LPIN1 C-terminal region12. These results suggest that LPIN1 has dual functions of transcriptional co-factor and phosphatidic acid phosphorylase. In fatty acid metabolism, PPARG can bind to retinoid-X-receptor (RXR) to regulate the expression of downstream genes13. PPAR/RXR is activated by ligands, and then binds to specific DNA sequences (PPAR response element, PPRE) in the promoter region of specific target genes, regulates gene transcription, and induces lipid metabolism and cell differentiation13. In the goat mammary epithelial cells, the PPARG gene can regulate the transcription of adipose differentiation-related protein (ADRP) and stearoyl-CoA desaturase 1 (SCD) genes by binding to PPRE of their promoter to promote milk fat synthesis14,15.
Although some functional roles of LPIN1 gene in dairy cow lactation have been clarified, the transcriptional regulation mechanism of LPIN1 gene remains unclear. Buffalo and cattle belong to different genera, and the solid content of buffalo milk is significantly higher than that of cattle milk, especially its milk fat content is almost twice that of cow milk16, suggesting that the genetic basis of lactation may be different between buffalo and dairy cows. So far, the specific role of LPIN1 gene in buffalo milk fat synthesis and its transcriptional regulation are still unknown. In this study, we hypothesized that LPIN1 plays an important role in the milk fat synthesis of buffalo mammary epithelial cells (BMECs), and that PPARG can regulate its transcription by binding to the potential PPREs in the promoter region of the buffalo LPIN1 gene. In order to confirm this hypothesis, LPIN1 knockdown by lentivirus-mediated interference and overexpression by transfecting recombinant fusion expression vector were performed in BMECs. Furthermore, we cloned the promoter of buffalo LPIN1 gene, and studied the transcriptional regulation mechanism of buffalo LPIN1 gene through the search of PPREs and the site-directed mutagenesis of this element, as well as the overexpression and interference of the PPARG gene. This study will provide strong evidence to clarify the role of LPIN1 in the synthesis of buffalo milk fat.
Results
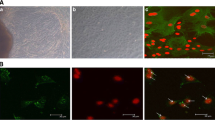
Identification of the BMECs
The BMECs were isolated by tissue block culture, which grew into a mixed population of cobblestone epithelial cells surrounded by spindle-shaped fibroblasts around them (Fig. 1A). After the mixed cells were purified three times with 0.25% trypsin EDTA, the fully purified island and cobblestone-like epithelial cells were obtained (Fig. 1B). Immunocytochemical detection showed that cytokeratin 18 were expressed in the epithelial cells (Fig. 1C, D), which revealed that the cells we obtained were BMECs.
Construction of LPIN1 overexpression vector and its effect on gene expression in the BMECs
The CDS of LPIN1 gene was first ligated to pEASY-T1 Simple Cloning vector, and then ligated to the pEGFP-C1 vector. The pEGFP-C1-LPIN1 and pEGFP-C1 were directly transfected into the BMECs, respectively. After 48 h, green fluorescence was observed under fluorescence inverted microscope (Leica, DMI4000B, Germany), which suggested that the pEGFP-C1-LPIN1 was successfully transfected into the BMECs (Fig. 2A). Compared with the control (pEGFP-C1), the mRNA abundance of LPIN1 in the BMECs transfected with pEGFP-C1-LPIN1 was significantly increased (25 fold) (P < 0.01; Fig. 2B). Meanwhile, the overexpression of LPIN1 significantly increased mRNA abundance of AGPAT6 (~ 3.4 fold), DGAT1 (~ 2.0 fold), DGAT2 (~ 4.4 fold), GPAM (~ 8.5 fold) and BTN1A1 (~ 2.9 fold) genes that involved in TAG synthesis (Fig. 2C). In addition, the overexpression of LPIN1 gene also significantly increased the expression of PPARG (~ 4.5 fold) and SREBF1 (~ 13.5 fold) genes related to the regulation of fatty acid metabolism, but had no significant effect on the expression of SREBF2 (P > 0.05; Fig. 2C).
Effect of LPIN1 overexpression in the BMECs. (A) Fluorescence results of LPIN1 overexpression in the BMECs. (A1). Green fluorescence of control group (pEGFP-C1); (A2) Ordinary light of control group (pEGFP-C1); A3. Green fluorescence of pEGFP-C1-LPIN1; A4. Ordinary light of pEGFP-C1-LPIN1; (B) The efficiency of LPIN1 overexpression; (C) The overexpression of LPIN1 up-regulates the expression of genes related to milk fat synthesis in the BMECs; (D) Changes of total TAG content in the BMECs under the LPIN1 overexpression. Values are displayed as means ± SEM for three biological replicates. *, P < 0.05; **, P < 0.01.
In order to determine whether the expression of LPIN1 affects the accumulation of TAG in the BMECs, the intracellular TAG content was measured using the TAG kit, and the results showed that the content of TAG in the pEGFP-C1-LPIN1 group was significantly higher than that in the pEGFP-C1 group (P < 0.01; Fig. 2D).
Construction of LPIN1 interference vector and its effect on gene expression in the BMECs
Three pairs of shRNAs were ligated into the PLKO.1 vector to construct recombinant plasmids (pLKO.1-shRNAs) for interfering with LPIN1 gene. The pLKO.1-shRNA and pEGFP-C1-LPIN1 vectors were co-transfected into HEK-293 T cell, and the green fluorescence results showed that the transfection efficiency could meet the experimental requirements (Fig. 3A). Compared with pEGFP-C1-LPIN1 group, the expression level of LPIN1 in the cells co-transfected with pEGFP-C1-LPIN1 and LPIN1-sh1, LPIN1-sh2, LPIN1-sh3 decreased by 46%, 82% and 63%, respectively. It shows that LPIN1-sh2 has the highest efficiency of LPIN1 knockdown (Fig. 3B).
Effect of LPIN1 interference in the BMECs. (A) The image of green fluorescence to identify transfection efficiency of shRNAs in HEK-293-T cells; A1. The image of ordinary light of co-transfected with pLKO.1-shRNA and pEGFP-C1-LPIN1; A2. The image of green fluorescence of co-transfected with pLKO.1-shRNA and pEGFP-C1-LPIN1; (B) LPIN1 interference efficiency of three shRNA recombinants evaluated via RT-qPCR; (C) Interference of LPIN1 down-regulates the expression of genes related to milk fat synthesis in the BMECs; (D) The effects of LPIN1 interference on the total cellular TAG content in the BMECs. Values are expressed as means ± SEM for three biological replicates. *P < 0.05; **P < 0.01.
The LPIN1-sh2 was selected to prepare lentivirus particles (Lv-LPIN1-sh2) and further transfected into BMECs. The results showed that the mRNA abundance of LPIN1 in the Lv-LPIN1-sh2 group was significantly lower than that in control (50%) (P < 0.01; Fig. 3C). The knockdown of LPIN1 significantly decreased the mRNA abundance of genes related to milk fat synthesis in the BMECs (Fig. 3C), and the relative expression of AGPAT6, DGAT1, DGAT2, GPAM and BTN1A1 genes involved in TAG synthesis decreased by 73%, 67%, 29%, 68% and 33%, respectively. Meanwhile, the knockdown of LPIN1 also decreased the transcriptional levels of genes involved in regulating fatty acid metabolism. For example, the expression of PPARG and SREBF1 was reduced by 22% and 83%, respectively, but it had no significant effect on the expression of SREBF2 (Fig. 3C).
Correspondingly, compared with the control group, the TAG content of the BMECs in the Lv-LPIN1-sh2 group was significantly reduced (P < 0.01) (Fig. 3D).
Characterization and activity analysis of buffalo LPIN1 promoter
We obtained a sequence of 1920 bp at the 5’ end of the buffalo LPIN1 gene by PCR, which contains 1878 bp upstream of the transcription start site (TSS) (+ 1) (Fig. 4A). Bioinformatics analysis showed that the G + C content of the amplified sequence was as high as 56.67%, containing a GC box (− 11 bp to − 19 bp), but not a typical TATA box. The sequence also contained several important cis-acting elements: PPAR response elements (PPRE1: − 481 bp to − 493 bp; PPRE2: − 86 bp to − 100 bp), sterol regulatory element (SRE: − 691 bp to − 700 bp) and the sites of nuclear factor (NF-Y: − 709 bp to − 715 bp), C/EBPα (− 408 bp to − 425 bp) and cAMP-response element binding protein (CREB: − 827 bp to − 835 bp) (Fig. 4B). The analysis by Methprimer software showed that the amplified sequence also contains a CpG island (Fig. 4C).
The pGL4-LPIN1 luciferase recombinant vector containing LPIN1 gene promoter (− 1878 bp to + 42 bp) was constructed, then transfected into the BMECs to determine the luciferase activity. Compared with the control (pGL4.11), the luciferase activity in the BMECs in the pGL4-LPIN1 group was significantly increased (~ 62 fold), indicating that the − 1878 to + 42 region of LPIN1 gene contains the promoter region of this gene (Fig. 4D; P < 0.01).
Identification of the core promoter region of LPIN1 gene
A series of luciferase reporter recombinant vectors with 5’ flanking deletion fragments of the LPIN1 promoter were constructed (− 1523/ + 42, − 1264/ + 42, − 953/ + 42, − 666/ + 42, − 416/ + 42, − 336/ + 42, − 172/ + 42 and − 88/ + 42). After transfecting these recombinant vectors into BMECs, luciferase activity was detected (Fig. 5). The deletion from − 1878 bp to − 1523 bp showed a decrease of luciferase activity that was trending toward significance (P = 0.07); when the deletion reached the − 1264 bp, the luciferase activity was significantly increased (P < 0.01); When the deletion reached − 953 bp, the luciferase activity was significantly reduced (P < 0.01), indicating that there are negative regulatory elements in the region from − 1264 bp to − 953 bp. In addition, the deletion from − 953 bp to − 666 bp has the highest luciferase activity (P < 0.01), and the deletion from − 666 bp to − 416 bp reduced the luciferase activity by about 56% (P < 0.01). Subsequently, with the deletion of the 5’ flanking region, the activity continued to decline (Fig. 5). This indicates that the fragment between − 666 bp and + 42 bp contains cis-functional elements required for transcriptional activation of LPIN1 gene. The above results reveal that the core region of the LPIN1 promoter is located in the region from − 666 bp to + 42 bp, which has basic transcriptional activity.
Luciferase activity of 5’-flanking fragment vectors of buffalo LPIN1 promoter in the BMECs. Relative luciferase activity (Firefly: Renilla) after 48 h transfection with different 5’-flanking deletion constructs (− 1878/ + 42 bp, − 1523/ + 42 bp, − 1264/ + 42 bp, − 953/ + 42 bp, − 666/ + 42 bp, − 416/ + 42 bp, − 336/ + 42 bp, − 172/ + 42 bp and − 88/ + 42 bp). **, P < 0.01.
Overexpression of PPARG increased the activity of LPIN1 promoter
In order to determine whether PPARG has a regulatory effect on the LPIN1 gene promoter, the pGL4-LPIN1 vector or 5’ deletion mutagenesis constructs were transfected with pEGFP-C1-PPARG to detect their luciferase activity. The results showed PPARG gene was successfully overexpressed in the BMECs, and its expression level was 540 times that of the control (pEGFP-C1) (Fig. 6A). In addition, the overexpression of PPARG significantly increased the mRNA expression of LPIN1 gene (~ 6.8 fold; Fig. 6B). Compared with the control (pEGFP-C1), after overexpression of PPARG (pEGFP-C1-PPARG), the activity of the 5’ deletion mutagenesis constructs was significantly increased except for the deletion fragment − 88/ + 42 (Fig. 6C). Therefore, it is speculated that there are PPRE elements in the LPIN1 promoter, and PPARG may regulate the expression of the LPIN1 gene by combining with the PPRE elements.
The effects of PPARG overexpression on the LPIN1 mRNA expression and promoter activity in the BMECs. (A) The mRNA expression of PPARG after transfection with pEGFP-C1-PPARG; (B) The mRNA expression of LPIN1 after transfection with pEGFP-C1-PPARG; (C) Relative luciferase activity (Firefly: Renilla) after 48 h transfection of pEGFP-C1-PPARG and different 5’ flanking deletion constructs (− 1878 bp/ + 42 bp, − 666/ + 42 bp, − 416/ + 42 bp, − 336/ + 42 bp, − 172/ + 42 bp and − 88/ + 42 bp. Values are provided as mean ± SEM; *P < 0.05; **P < 0.01.
Interference of PPARG decreased the activity of LPIN1 promoter
After the shRNA mediated-knockdown of PPARG was conducted in BMECs, the mRNA expression of LPIN1 and PPARG and the luciferase activity of each promoter fragment were measured. The results showed that after interference with the PPARG gene (Lv-PPARG-sh3), the mRNA level of this gene was significantly reduced (P < 0.01; Fig. 7A), and the mRNA level of the LPIN1 gene also decreased significantly (P < 0.01; 43%; Fig. 7B). In addition, compared with the control (Lv-NC), after interfering with PPARG, except for that of the − 88 bp/ + 42 bp fragment vector, the luciferase activity of all the 5’ deletion fragment vectors decreased significantly (Fig. 7C). This result further confirms that there are PPARG binding target sites (PPREs) in the LPIN1 promoter, and PPARG may directly regulate its expression by binding to the PPREs on the promoter of LPIN1 gene.
Effects of PPARG knockdown on LPIN1 promoter activity in the BMECs. (A) The mRNA expression of PPARG after treatment with Lv-PPARG-sh3; (B) The mRNA expression of LPIN1 after treatment with Lv-PPARG-sh3; (C) Relative luciferase activity (Firefly: Renilla) of 5’ flanking deletion constructs (− 1878 bp/ + 42 bp, − 666/ + 42 bp, − 416/ + 42 bp, − 336/ + 42 bp, − 172/ + 42 bp and − 88/ + 42 bp). Values are provided as mean ± SEM; *, P < 0.05; **, P < 0.01.
Site-directed mutation of the PPRE decreased basal activity of the LPIN1 promoter
To determine whether PPARG regulates the expression of the gene by binding to the two predicted PPREs (PPRE1 and PPRE2) of the LPIN1 promoter, recombinant vectors with the site-directed mutations on the PPREs located in the core promoter region of LPIN1 (− 666/ + 42 bp) were constructed, respectively, including the recombinants for site-directed mutagenesis of PPRE1 and PPRE2, and vector for simultaneous mutagenesis of both. Luciferase detection showed that both PPRE1 and PPRE2 site-directed mutagenesis could significantly decrease the promoter activity of LPIN1 gene. The decrease of LPIN1 promoter activity after site-directed mutation of PPRE1 (62%) was greater than that after site-directed mutation of PPRE2 (44%) (Fig. 8). In addition, after the simultaneous site-directed mutation of PPRE1 and PPRE2, the LPIN1 promoter activity decreased to a greater extent compared to PPRE1 or PPRE2 mutation alone (82%) (Fig. 8). It is suggested that PPARG can directly regulate the transcription of LPIN1 gene by binding to the PPRE1 and PPRE2, and the PPRE1 plays a more important role in the binding of PPARG.
Discussion
The mammary gland of lactating cows is a formidable TAG synthesis machine7. The synthesis of milk fat in the mammary gland includes biological processes such as uptake and transport of long-chain fatty acids, de novo synthesis of fatty acids, desaturation, synthesis of triglycerides, formation and desaturation of lipid droplets. Milk fat synthesis is regulated by PPARG, SREBF and other transcription factors17. The lipid droplets formed in the endoplasmic reticulum of BMECs are released into the acinar cavity of the mammary gland in the form of milk fat globules18. In the TAG synthesis pathway, glycerol-3-phosphate acyltransferase (GPAM) first converts glycerol-3-phosphate (G-3-P) and fatty acyl-CoA into a lysophosphatidic acid (LPA) in the endoplasmic reticulum. Then, LPA is converted into a phosphatidic acid (PA) by the AGPAT enzyme. PA is subsequently dephosphorylated by the enzyme LPIN1 to form a diacylglycerol (DAG). Then, DGAT makes DAG plus fatty acid acyl-CoA to form a TAG, which is the rate-limiting enzyme for TAG synthesis19. As a member of the immunoglobulin superfamily, butyrophilin A1 (BTN1A1) is essential for regulating the secretion of milk fat droplets20. In this study, the functional role of LPIN1 in buffalo milk fat synthesis was investigated by lentivirus-mediated interference and overexpression experiments in vitro. The overexpression of LPIN1 gene led to a significant increase in mRNA expression of GPAM, DGAT1, DGAT2 and BTN1A1 genes involved in TAG synthesis and secretion in the BMECs, as well as a significant increase in TAG content (Fig. 2). On the contrary, after the knockdown of LPIN1 gene, the expression of these genes in the BMECs was decreased, and the TAG content was also significantly decreased (Fig. 3). This suggests that buffalo LPIN1 gene plays an important role in the triglyceride pathway of buffalo milk fat synthesis, and it is speculated that this gene also participates in the synthesis of TAG in BMECs by catalyzing the dephosphorylation of PA to form DAG. Previous studies in goat mammary epithelial cells showed that knockdown of PPARG gene led to a significant decrease in LPIN1 gene, suggesting that LPIN1 gene may be a downstream target gene of PPARG during milk fat synthesis in ruminants14. Our previous research revealed that PPARG is a central regulator of buffalo milk fat synthesis21. Another study found that LPIN1, as a transcription co-activator, its C-terminal amino acid residues at 217–399 can enhance the transcriptional activation activity of PPARG12. In this study, the expression of PPARG gene was significantly increased after the overexpression of LPIN1 gene (Fig. 2). Moreover, the overexpression of PPARG gene also significantly increased the expression of LPIN1 gene (Fig. 6). Therefore, it was speculated that in the BMECs, the LPIN1 gene involved in milk fat synthesis may be regulated by the PPARG. Meanwhile, LPIN1 may also activate PPARG through transcriptional co-activation, and then PPARG regulates the milk fat synthesis of BMECs, which needs further confirmation in subsequent experiments.
SREBF1 is considered to be an important central regulator in the regulatory network of milk fat synthesis22,23. Previous studies have shown that SREBF1 gene is a target gene of PPARG24. Based on the fact that the expression of SREBF1 gene was significantly down-regulated after LPIN1 interference in this study (Fig. 3), it is speculated that the interference of LPIN1 gene initially reduced the expression of PPARG, which further led to the down-regulation of SREBF1 expression. The main function of SREBF2 is to specifically regulate cholesterol synthesis22. In this study, the overexpression or interference of LPIN1 gene had no significant effect on the expression of SREBF2, suggesting that LPIN1 gene may not be involved in the synthesis of cholesterol in BMECs.
Study has shown that the expression of LPIN1 gene is regulated by cholesterol, and SREBF1 can directly bind to the sterol regulatory element (SRE) on the LPIN1 promoter to promote the transcription of this gene25. In humans and mice, LPIN1 is regulated by a variety of transcription factors, including SREBF126, estrogen-related receptor γ (ERRγ)27, hepatic nuclear factor 4a (HNF4a)28 and C/EBPα6. They directly bind to the corresponding sites in the LPIN1 promoter to promote LPIN1 transcription and lipid synthesis. In this study, not only the binding sites of SREBF1 and C/EBPα were found in the promoter of the buffalo LPIN1, but also the PPREs sites. In the fatty acid synthesis pathway, PPARG binds to RXR to form a heterodimer, which then binds to the ligand and enters the nucleus, and then recognizes the PPREs on the target gene promoter, thus activating the transcription of downstream genes13,29. We found two PPREs (PPRE1: TGGCCTTGTGACAG; PPRE2: GGGTCGAAAGATCT) in the promoter of buffalo LPIN1, which were highly consistent with the recognized PPRE13. So far, PPRE has been found in the promoter of many genes related to lipid synthesis, such as thrombospondin receptor (CD36)30, adipose differentiation-related protein (ADRP) and stearoyl-CoA desaturase 1 (SCD)15. In this study, after the overexpression of PPARG, the luciferase activity of all recombinants with 5’ deletion fragments of the LPIN1 promoter increased significantly except for fragment − 88/ + 42 (Fig. 6). In contrast, after interference with PPARG, the luciferase activity of all recombinants with 5’ deletion fragments of the LPIN1 promoter was significantly decreased except for fragment − 88/ + 42 (Fig. 7). Furthermore, the site-directed mutagenesis of PPRE1 and PPRE2 in the core promoter of LPIN1 greatly reduced the transcriptional activity of the promoter fragment, and after the simultaneous site-directed mutagenesis of PPRE1 and PPRE2, the activity of the core promoter was significantly lower than that of PPRE1 and PPRE2 alone (Fig. 8). The results here indicate that PPARG can directly regulate the transcription of buffalo LPIN1 by binding to the PPRE1 and PPRE2 sites on its promoter.
Materials and methods
Ethics declarations
Sample collection in the described experiments was approved by the Animal Care and Use Committee of Yunnan Agricultural University (No. YNAU2019llwyh019). Every effort has been made to minimize suffering. All methods were performed in accordance with the relevant guidelines and regulations and the study is reported in accordance with ARRIVE guidelines.
Isolation, culture and identification of buffalo mammary epithelial cells
BMECs were isolated, purified and cultured from buffalo mammary gland tissue at the peak of lactation using the previously described method21. The female buffalo used for sampling (Binglangjiang buffalo, an indigenous river type buffalo breed distributed in western Yunnan Province, China) was healthy, five years old and at the 3rd parity (about 60 d postpartum). In short, the fresh buffalo mammary tissue was collected and washed with the phosphate buffer saline (PBS, Gibco, USA) for three times. Part of the acini was washed by highly resistant PBS (containing 400 IU mL-1 penicillin and 400 IU mL-1 streptomycin). The tissue blocks were then cut into 1 to 2 mm3 pieces and spread at the petri dishes, then cultured in an incubator with the condition of 37 °C, 5% CO2 and 95% air humidity. The culture medium is DMEM/F12 (Gibco, USA) supplemented with fetal bovine serum (10%, Gibco, USA), penicillin/streptomycin (10 kU/L, Gibco, USA), bovine insulin (5 mg/L, Sigma, USA), hydrocortisone (5 mg/L, Sigma, USA), epidermal growth factor (1 mg/L, Sigma, USA) and 5 μg/mL holotransferrin (Sigma, USA). The cells began to grow about 12 days. The cells were digested with 0.25% trypsin-ethylenediamine tetraacetic acid (EDTA, Gibco, USA) for 2–3 min, and the exfoliated fibroblasts were then washed with the PBS. The remaining cells adhered to the bottom of the petri dish were further digested for 5–10 min and centrifuged at 1250 rpm for 3 min. The purified cells were collected for subculture. After 3 times of purification, the purified BMECs could be obtained. The identification protocol of BMECs is as follows: When the purified BMECs were confluent with 70–80%, the cells were washed with 1 × PBS, fixed with 4% paraformaldehyde for 20 min, then incubated 30 min with 1 × BSTX (1 × PBS containing 10% BSA, 0.1% Triton X-100) (Sigma, USA) for blocking. The cells were then incubated with 1:100 diluted cytokeratin 18 antibody (Sigma, USA) for 1 h, then were washed for three times and incubated with FITC-labeled monoclonal anti-mouse IgG secondary antibody (Sigma, USA) at 1:100 dilutions for 1 h. In the end, the purified cells were taken images with a microscope (Leica, DMI4000B, Germany).
Construction of vector for overexpression and interference of LPIN1 gene
Based on the mRNA sequence of buffalo LPIN1 (XM_006073743), a pair of primers were designed using Primer Premier 5.0 software31: LPIN1-sense, 5’-AAGCTTATGAACTACGTCGGGCAG-3’ (Hind III restriction endonuclease site was underlined); LPIN1-antisense, 5’-GGTACCCTAGGCTGATGCAGAATGAAC-3’ (KpnI restriction endonuclease site was underlined). The PCR product was purified by a Gel Extraction Kit (OMEGA, USA), and then cloned into pEASY-T1 Simple Cloning vector (TransGen, China). Subsequently, the target fragment was ligated into the pEGFP-C1 (Takara, China) vector to construct an overexpression recombinant vector, referred as pEGFP-C1-LPIN1. Then, the positive recombinants were purified using the EndoFree Maxi Plasmid Kit (QIAGEN, Germany) for subsequent cell transfection experiment. The sequencing verification was completed by Shanghai Biological Engineering Technology Services Co., Ltd. (Shanghai, China).
According to the mRNA sequence of buffalo LPIN1 gene (XM_006073743), three pairs of short hairpin RNAs (shRNAs) targeting the LPIN1 gene were designed using the online software BLOCK-iT RNAi Designer (http://rnaidesigner.invitrogen.com/rnaiexpress/). After the shRNA sequences were annealed, it was ligated into the pLKO.1 vector to generate final recombinant plasmids (pLKO.1-shRNAs). All the recombinant vectors were sequenced for verification and then purified using EndoFree Maxi Plasmid Kit (QIAGEN, Germany). The sequences of shRNAs are presented in Table 1.
Overexpression of buffalo LPIN1 gene
When the confluence of BMECs in a 6-well plate is 70–80%, pEGFP-C1-LPIN1 was transfected into the BMECs using Lipo6000™ transfection reagent (Beyotime Biotechnology, China) in accordance with the manufacturer’s protocol, and pEGFP-C1 was transfected as negative control. Forty-eight hours after transfection, the green fluorescent protein (GFP) was monitored using a Leica fluorescent microscope (DMI4000B, Germany). Then, the BMECs were harvested for total RNA extraction or triacylglycerol (TAG) content analysis.
Knockdown of buffalo LPIN1 gene
HEK-293 T cells (purchased from Kunming Institute of Zoology, Chinese Academy of Sciences) were cultured in DMEM/F12 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin (10 kU/L, Gibco, USA). HEK-293 T cells with a confluence rate of 70–80% were co-transfected with pLKO.1-shRNA and pEGFP-C1-LPIN1 at a ratio of 3:1 to screen for the most effective shRNA targeting the LPIN1 gene. The pEGFP-C1-LPIN1 vector was transfected alone as a control. Forty-eight hours after transfection, the expression of GFP was monitored using a Leica fluorescent microscope (DMI4000B, Germany). The HEK-293 T cells were then collected for RT-qPCR analysis. The PLKO.1 system used for the RNAi was a gift from David Root Lab32. When HEK-293 T cells were cultured to 70–80% confluence in a 10 cm plate, Lipo6000 (Beyotime Biotechnology, China) was used to co-transfection pLKO.1-shRNA, pMD2G (enveloped plasmid) and pPAX2 (packaged plasmid) into HEK-293 T cells at a ratio of 5:3:2 to produce lentivirus (Lv-LPIN1-shRNA). In addition, pLKO.1, pMD2G and psPAX2 were co-transfected into HEK-293 T cells as a negative control group (Lv-NC). The culture medium contained the lentivirus was centrifuged by 400 g for 5 min at 4 °C by a high-speed freezing centrifuge (Sorvall ST8; Thermo Fisher Scientific, USA) and then filtered with a 0.45 μm filter. The lentivirus particle was stored at − 80 °C for long-term storage.
When the BMECs were cultured in a 60 mm plate to 70–80% confluence, the lentivirus (Lv-LPIN1-shRNA or Lv-NC) was added to culture medium. Meanwhile, 2 mL of polybrene (2 μg/mL, Sigma, USA) was added to the culture medium to improve the efficiency of virus infection. The medium was replaced with fresh medium after 24 h. The BMECs were harvested after 48 h for RNA extraction or TAG content analysis. Since PLKO.1 vector does not express fluorescent protein, multiplicity of infection (MOI) was not estimated in this study, and only RT-qPCR was used to ensure sufficient interference efficiency.
Gene expression detection
Total RNA was extracted and purified from the BMECs, and the cDNA was synthesized using a PrimeScript RT kit (TaKaRa, China). RT-qPCR primer pairs spanning exon-exon were designed with Primer Premier 5.0 to avoid amplification of genomic DNA. Detailed information about the primer pairs is present in Table 2. The qPCR was performed an Applied Biosystems™ 7500 (Thermo Fisher, USA) with TB Green® Advantage® qPCR Premix (Takara, China). The efficiency of amplification was determined using program LinRegPCR (https://www.medischebiologie.nl/files/?main=files&fileName=LinRegPCR.zip&description=LinRegPCR:%20qPCR%20data%20analysis&sub=LinRegPCR). The ubiquitously expressed genes of ACTB (actin beta), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and YWHAZ (tyrosine 3-monooxygenase tryptophan 5-monooxygenase activation protein zeta) were used as internal reference genes to normalize the target gene expression21. The genes for expression detection here included the PPARG, glycerol-3-phosphate acyltransferase (GPAM), diacylglycerol acyltransferase 1 (DGAT1), diacylglycerol acyltransferase 2 (DGAT2), 1-acylglycerol-3-phosphate O-acyltransferases6 (AGPAT6), lipid phosphate phosphohydrolase1 (LPIN1), butyrophilin A1 (BTN1A1), sterol regulatory element-binding transcription factor 1 (SREBF1) and sterol regulatory element-binding transcription factor 2 (SREBF2). They are associated with the synthesis and regulation of milk fat33. The mRNA abundance of these genes was quantified using the 2-ΔΔCt method. The ΔCt = Ct (target genes)-Ct (reference genes). ΔΔCt = ΔCt-ΔCt (control). The control in the formula was group Lv-NC in the knockdown experiment, and pEGFP-C1 in gene overexpression experiment. Finally, the ACTB, GAPDH and YWHAZ were used as internal reference genes to calculate the 2-ΔΔCt of each gene, and then the geometric mean of the three 2-ΔΔCt values for each gene was calculated as the gene expression.
TAG content assay
After the overexpression or knockdown of LPIN1 for 48 h, the BMECs were rinsed twice with PBS. The TAG content in the BMECs was determined using the TAG kit (GPO-POD; Applygen Technologies Inc., Beijing, China) following the manufacturer’s instructions. Meanwhile, the intracellular total protein content was measured using the BCA protein assay kit (Thermo Fisher Scientific, USA). The TAG content was normalized by per milligram of protein.
Cloning and bioinformatics analysis of buffalo LPIN1 promoter
Genomic DNA was extracted from the blood samples of Binglangjiang buffalo (adult, healthy) using the method of phenol/chloroform purification-based protocol. A pair of primers named LPIN1_F/R were designed to conduct the PCR for isolating the promoter of the LPIN1 (Table 3). Amplified products were detected by 1.5% agarose gel electrophoresis. The target bands were excised from the agarose gel and then purified by Gel Extraction Kit (OMEGA, China). The purified PCR products were cloned into pMD18-T vector (TaKaRa, China) and further bidirectionally sequenced. Prediction of transcription factor binding sites in the promoter sequence was executed using online software JASPAR database (http://jaspar.genereg.net/) and Peroxisome Proliferator Response Elements Search (http://www.classicrus.com/PPRE/).
Construction of luciferase reporter gene vector
The complete buffalo LPIN1 promoter fragment was excised from the pMD18-T vector with Xho I and Hind III restriction enzymes (TaKaRa, China), and then ligated into the pGL4.11 vector to generate the final promoter recombinant plasmid (pGL4-LPIN1). In addition, a series of upstream primers were designed using the complete promoter region sequence of LPIN1 gene as a template, the following 5’ flanking deletion fragments of the promoter were further obtained by PCR: − 1523/ + 42, − 1264/ + 42, − 953/ + 42, − 666/ + 42, − 416/ + 42, − 336/ + 42, − 172/ + 42 and − 88/ + 42 (Table 3). The recombinant vectors of these 5’ flanking deleted promoter fragments were constructed, and all the fragments inserted into the vectors were confirmed by bidirectional sequencing. Then, the overlapping PCR method was used to generate site-directed mutagenesis with TIANSeq HIFI Amplification Mix (QIAGEN, Germany). The specific primers of overlapping PCR for site-directed mutagenesis promoters are shown in Table 3. All the PCR products were subsequently cloned into pGL4.11 vector, and all recombinant vectors were confirmed by DNA sequencing.
Luciferase-based assays
When the confluence of BMECs in 12-well plates was 70–80%, the pGL4-LPIN1 vector or all recombinant vectors with 5’ deletion mutagenesis were co-transfected with pRL-TK vector in a ratio of 10:1 using Lipo6000™ transfection reagent (Beyotime Biotechnology). The pGL4.11 vector was co-transfected with pRL-TK as negative control. After 48 h of culture, the BMECs were collected for the determination of luciferase activity. The lentivirus (Lv-PPARG-sh3) used for PPARG gene knockdown and pEGFP-C1-PPARG used for overexpression of PPARG gene were constructed by our laboratory in the previous research21. In order to investigate the effect of PPARG overexpression and knockdown on the activity of the LPIN1 promoter, after the BMECs were transfected with Lv-PPARG-sh3 or pEGFP-C1-PPARG for 12 h, the pGL4-LPIN1 plasmid and 5’deletion recombinant constructs of LPIN1 promoter were transiently co-transfected the BMECs from the previous step with pRL-TK, and the BMECs were collected for RT-qPCR analysis and luciferase activity analysis after 48 h of culture. The BMECs were washed twice with cold PBS and luciferase activity was measured by Dual-Luciferase® Reporter Assay System (Promega, Wisconsin, USA). The relative luciferase activity of each sample was normalized with pRL-TK.
Statistical analysis
Three repetitions were set for all treatments, and the results were expressed as mean ± standard error of means (means ± SEM). Statistical comparisons (t-test) between the treatment and the control group were performed using software SPSS 19.0 (SPSS Inc., Chicago, IL) with a significance level of 0.05 and an extremely significant level of 0.01.
Conclusion
In this study, in vitro overexpression and interference experiments showed that the interference with LPIN1 significantly reduced the expression of lipid synthesis related genes and TAG content in the BMECs, while the overexpression of LPIN1 significantly increased the expression of lipid synthesis related genes and TAG content in the BMECs. Further transcriptional regulation experiments showed that the overexpression or interference of PPARG could enhance or decrease the expression and promoter activity of buffalo LPIN1, and the site-directed mutagenesis of PPRE1 and PPRE2 significantly reduced the promoter activity of buffalo LPIN1. The results here suggest that buffalo LPIN1 is involved in the synthesis of milk fat, and the PPARG can directly regulate the transcription of buffalo LPIN1 by binding to the PPRE1 and PPRE2 in its core promoter.
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
Péterfy, M., Reue, K., Phan, J. & Xu, P. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27, 121–124. https://doi.org/10.1038/83685 (2001).
Csaki, L. S. & Reue, K. Lipins: multifunctional lipid metabolism proteins. Annu. Rev. Nutr. 30, 257–272. https://doi.org/10.1146/annurev.nutr.012809.104729 (2010).
Reue, K. & Zhang, P. The lipin protein family: dual roles in lipid biosynthesis and gene expression. Febs Lett. 582, 90–96. https://doi.org/10.1016/j.febslet.2007.11.014 (2008).
Donkor, J., Sariahmetoglu, M., Dewald, J., Brindley, D. N. & Reue, K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282, 3450–3457. https://doi.org/10.1074/jbc.M610745200 (2006).
Phan, J., Péterfy, M. & Reue, K. Lipin expression preceding peroxisome proliferator-activated receptor-γ is critical for adipogenesis in Vivo and in Vitro. J. Biol. Chem. 279, 29558–29564. https://doi.org/10.1074/jbc.M403506200 (2004).
Koh, Y. et al. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ2. J. Biol. Chem. 283, 34896–34906. https://doi.org/10.1074/jbc.M804007200 (2008).
Bionaz, M. & Loor, J. J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J. Nutr. 138, 1019–1024. https://doi.org/10.1093/jn/138.6.1019 (2008).
Moyes, K. et al. Gene network and pathway analysis of bovine mammary tissue challenged with Streptococcus uberis reveals induction of cell proliferation and inhibition of PPARγ signaling as potential mechanism for the negative relationships between immune response and lipid metabolism. BMC Genom. 10, 542. https://doi.org/10.1186/1471-2164-10-542 (2009).
Lee, J. N. et al. Characterisation of gene expression related to milk fat synthesis in the mammary tissue of lactating yaks. J. Dairy Res. 84, 283–288. https://doi.org/10.1017/S0022029917000413 (2017).
Bugge, A., Grøntved, L., Aagaard, M. M., Borup, R. & Mandrup, S. The PPARgamma2 A/B-domain plays a gene-specific role in transactivation and cofactor recruitment. Mol. Endocrinol. 23, 794–808. https://doi.org/10.1210/me.2008-0236 (2009).
Kim, J. et al. PPARγ agonists induce adipocyte differentiation by modulating the expression of Lipin-1, which acts as a PPARγ phosphatase. Int. J. Biochem. Cell Biol. 81, 57–66. https://doi.org/10.1016/j.biocel.2016.10.018 (2016).
Kim, H. E. et al. Lipin1 regulates PPARγ transcriptional activity. Biochem. J. 453, 49–60. https://doi.org/10.1042/BJ20121598 (2013).
Chinetti, G., Fruchart, J. C. & Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 49, 497–505. https://doi.org/10.1007/s000110050622 (2000).
Shi, H. et al. PPARγ regulates genes involved in triacylglycerol synthesis and secretion in mammary gland epithelial cells of dairy goats. PPAR Res. 2013, 310948. https://doi.org/10.1155/2013/310948 (2013).
Yao, D. et al. SCD1 alters long-chain fatty acid (LCFA) composition and its expression is directly regulated by SREBP-1 and PPARγ 1 in dairy goat mammary cells. J. Cell. Physiol. 232, 635–649. https://doi.org/10.1002/jcp.25469 (2017).
D’Ambrosio, C. et al. A proteomic characterization of water buffalo milkfractions describing PTM of major species and theidentification of minor components involved in nutrientdelivery and defense against pathogens. Proteomics 8, 3657–3666. https://doi.org/10.1002/pmic.200701148 (2008).
Osorio, J. S., Lohakare, J. & Bionaz, M. Biosynthesis of milk fat, protein, and lactose: roles of transcriptional and posttranscriptional regulation. Physiol. Genomics 48, 231–256. https://doi.org/10.1152/physiolgenomics.00016.2015 (2016).
McManaman, J. L., Russell, T. D., Schaack, J., Orlicky, D. J. & Robenek, H. Molecular determinants of milk lipid secretion. J. Mammary Gland Biol. Neoplasia 12, 259–268. https://doi.org/10.1007/s10911-007-9053-5 (2007).
Wang, H., Airola, M. V., Reue, K. How lipid droplets “TAG” along: Glycerolipid synthetic enzymes and lipid storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 1131–1145, https://doi.org/10.1016/j.bbalip.2017.06.010 (2017).
Ogg, S. L., Weldon, A. K., Dobbie, L., Smith, A. J. & Mather, I. H. Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc. Natl. Acad. Sci. USA 101, 10084–10089. https://doi.org/10.1073/pnas.0402930101 (2004).
Zhou, F. T., Ouyang, Y. N. & Miao, Y. W. Peroxisome proliferator-activated receptor gamma regulates genes involved in milk fat synthesis in mammary epithelial cells of water buffalo. Anim. Sci. J. 92, e13537. https://doi.org/10.1111/asj.13537 (2021).
Eberle, D., Hegarty, B., Bossard, P., Ferre, P. & Foufelle, F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848. https://doi.org/10.1016/j.biochi.2004.09.018 (2004).
Xu, H. F. et al. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J. Dairy Sci. 99, 783–795. https://doi.org/10.3168/jds.2015-9736 (2016).
Kast-Woelbern, H. R. et al. Rosiglitazone induction of Insig-1 in white adipose tissue reveals a novel interplay of peroxisome proliferator-activated receptor gamma and sterol regulatory element-binding protein in the regulation of adipogenesis. J. Biol. Chem. 279, 23908–23915. https://doi.org/10.1074/jbc.M403145200 (2004).
Ishimoto, K. et al. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J. Biol. Chem. 284, 22195–22205. https://doi.org/10.1074/jbc.M109.028753 (2009).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420. https://doi.org/10.1016/j.cell.2011.06.034 (2011).
Kim, D. et al. Estrogen-related receptor γ (ERRγ) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J. Biol. Chem. 286, 38035–38042. https://doi.org/10.1074/jbc.M111.250613 (2011).
Chen, Z. J., Gropler, M. C., Mitra, M. S. & Finck, B. N. Complex interplay between the lipin 1 and the hepatocyte nuclear factor 4 α (HNF4α) pathways to regulate liver lipid metabolism. PLoS ONE 7, e51320. https://doi.org/10.1371/journal.pone.0051320 (2012).
Schoonjans, K., Staels, B. & Auwerx, J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1302, 93–109. https://doi.org/10.1016/0005-2760(96)00066-5 (1996).
Tontonoz, P., Nagy, L., Alvarez, J. G. A., Thomazy, V. A. & Evans, R. M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252. https://doi.org/10.1016/S0092-8674(00)81575-5 (1998).
Lalitha, S. Primer Premier 5. Biotech. Softw. Internet Rep. 1, 270–272. https://doi.org/10.1089/152791600459894 (2000).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298. https://doi.org/10.1016/j.cell.2006.01.040 (2006).
Bionaz, M. & Loor, J. J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 9, 366. https://doi.org/10.1186/1471-2164-9-366 (2008).
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant Nos. 31460582 and 31760659) and the Natural Science Foundation Key Project of Yunnan Province, China (Grant Nos. 2014FA032 and 2007C0003Z).
Author information
Authors and Affiliations
Contributions
F.Z. Writing original draft preparation. F.Z. and X.F. Performed the experiments and the data collection and analysis. X.F. and Y.M. Writing – review & editing. Y.M.: Project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, F., Fan, X. & Miao, Y. LPIN1 promotes triglycerides synthesis and is transcriptionally regulated by PPARG in buffalo mammary epithelial cells. Sci Rep 12, 2390 (2022). https://doi.org/10.1038/s41598-022-06114-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06114-w
- Springer Nature Limited
This article is cited by
-
Review: genetic background of milk fatty acid synthesis in bovines
Tropical Animal Health and Production (2023)