Abstract
The abdominal aortic aneurysm (AAA) anatomy influences the technical success of the endovascular aneurysm repair (EVAR), yet very few data regarding the aortic tree angles exist in the literature. This poses great limitations in the numerical analyses of endografts, constraining their design improvement as well as the identification of their operational limitations. In this study, a matrix Φ of 10 angles was constructed for the description of the pathological region and was implemented on a large dataset of anatomies. More specifically, computed tomography angiographies from 258 patients were analysed and 10 aortic angles were calculated per case, able to adequately describe the overall AAA shape. 9 dimensional variables (i.e. diameters and lengths) were also recorded. The median and extreme values of these variables were computed providing a detailed quantification of the geometrical landscape of the AAA. Moreover, statistical analysis showed that the identified angles presented no strong correlation with each other while no lateral or anterior/posterior symmetry of the AAA was identified. These findings suggest that endograft designers are free to construct any extreme case-studies with the values provided in a mix-and-match manner. This strategy can have a powerful effect in EVAR stent graft designing, as well as EVAR planning.
Similar content being viewed by others
Introduction
Abdominal aortic aneurysm (AAA) is the permanent and irreversible dilatation of the lower section of the aorta. In AAAs, the vessel expands becoming susceptible to rupture, a highly dangerous incident which may lead to death. Prevention or emergent intervention of rupture has been conducted for decades via open surgery but in recent years the approach of endovascular aneurysm repair (EVAR) is preferred1.
In clinical practice, several methods for defining, grading and evaluating the aneurysmal site have been proposed2,3,4,5,6, but with small variations, all of them take into account the following anatomical characteristics:
- 1.
Proximal aortic neck
morphology (straight, taper, reverse taper or bulging)
length
diameter
angle between the flow axis of the neck and the body of the aneurysm
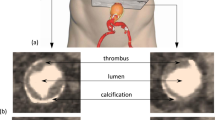
amount of thrombus and calcification
- 2.
Aneurysm
maximum AAA diameter
tortuosity
most acute aortic angle
amount of thrombus and calcification
- 3.
Distal aortic neck
length
diameter
- 4.
Iliac arteries (common, internal, external)
diameter
evaluation of the presence of stenosis/occlusion
sealing zone length
tortuosity
most acute aortic angle
amount of thrombus and calcification.
Apart from the aneurysm and the aortic necks, which are self-evidently necessary to be examined, iliacs are also included in all reports, because when ill-shaped they can be an exclusion criteria for EVAR. While the overall most common factor of EVAR disqualification is regarded to be the length and anatomy of the proximal AAA neck7,8, it is calcification, occlusion and tortuosity of the iliac arteries that are responsible for the majority of access complications during EVAR procedures9. Despite that, quantification of the angles involved in the AAA is rarely performed in clinical routine9. According to Henretta et al.10, the difficult anatomy of the iliac arteries can lead to some form of complication (injury of the arteries, misalignment of the stent-graft during deployment etc.) in up to 47% of patients whereas according to Clough et al.11, up to 17% of all EVAR procedures can result in significant problems.
Nowadays, it is common knowledge that AAA anatomy influences EVAR technical success, endoleak rate, migration of the endograft, as well as the need for secondary interventions4. It is for these reasons that the identification of average and extreme/worst case values for the AAA geometry is paramount, in order to challenge endograft designs in experimental set-ups, before they even reach the operating theatre. Nevertheless, similar to clinical practice, only a few angles are discussed in the literature, while global metrics like the tortuosity index cannot describe the geometry in a unique way that can allow its recreation.
Numerical analyses that try to establish how the variability in aortic geometry affects the endografts, or whether specific aortic angles result in endovascular complications, have to assume ranges of values for variables ill-documented. Li et al. reported the aortic neck angle doesn’t affect the migration force of the stent when smaller than 30°, yet for values above 40°, the force can almost triple12. They also demonstrated that the neck angle significantly influences the blood flow of an unstented AAA. Similarly, Morris et al.13 reported that increase in the aortic bifurcation angle increases the stent graft’s drag force by up to 50%, without establishing how common the angle values they used are. Furthermore, these parametric studies usually occur in a single plane, meaning that complex aortic angle changes in realistic 3D structures are avoided.
In this study, a series of measurements and statistical analyses were made based on a large dataset of EVAR patients. The aim was to quantify an extended set of geometrical variables for the AAA. These results can help stent graft investigators build both representative and challenging case studies for the assessment and improvement of endograft devices. In addition, the methodology presented for the description of the AAA shape can be used as a measurement protocol during EVAR planning, aiding to the identification of the most appropriate endograft given the arterial geometry of a patient and the endografts’ optimum operational range.
Methods
Study population and data acquisition
A series of data provided by M2S, Inc. West Lebanon, NH, USA were used to examine a range of aortic characteristics (Fig. 1). Computed tomography angiography (CTA) scans were originally collected during the “Vascutek Anaconda stent graft system phase II IDE study” in the period May 2009 to July 2011 in various sites in the United States and Canada. The study was approved by the U.S. Food and Drug Administration (registration number: NCT00612924 in the U.S. National Library of Medicine14) in accordance with relevant regulations. All patients provided their informed consent. Those data were supplied to us anonymised, with age and gender being the only descriptors of the patients.
3D reconstruction of the aortic geometry from 2D CTA scans in the m2s Preview. The blood lumen (red), the calcified regions (white) and the thrombus (transparent yellow) are visible. The dot at the top left corner corresponds to the (0,0,0) point of the coordinate system, used for all measurements. It is located at the posterior right corner of the 1st CTA scan.
The study included 258 patients (222 (86%) men, 34 (13%) women and 2 (1%) unspecified) aged between 51 and 88 years old. It was a non-randomized, continuous enrolment study that included patients treated with the AnacondaTM stent graft (Terumo Aortic). Patients provided their consent for the use of their data and the inclusion criteria for them were:
Infrarenal AAA ≥ 4.0 cm in diameter, or AAA growth ≥ 1.0 cm/year
Iliac artery distal fixation sites ≥ 20 mm in length
Ability to preserve at least one internal iliac artery
Femoral/Iliac artery’s size and morphology should be compatible with the appropriate delivery system (18 F, 20 F or 23 F).
Pregnant patients, or patients with thrombus, calcification and/or plaque ≥ 2 mm in thickness and/or 50% continuous coverage of the vessel’s circumference in the intended fixation site were excluded. Among the exclusion criteria were also aneurysms that extended above the renal arteries, significant (>80%) renal artery stenosis not readily treatable, previous AAA repairs and ruptured or leaking AAAs.
The follow up period of the study was 5 years, but herein only pre-op data were used.
Aortic angles
In the process of describing the AAA shape, a set of 10 angles was used to accurately describe the pathological region, using the m2s Preview v4.0.1 software. More specifically, following the centreline of the vascular tree, 14 points were identified as critical to describe the AAA geometry (Fig. 2 and Table 1). After qualitatively studying a series of AAAs, these points were considered both strategic and adequate to “depict” the overall AAA shape. Note that since tortuosity at the aneurysmal region is very rare15, no points in the aneurysmal sac were considered. Furthermore, even if the aneurysm does exhibit such an angle, the endograft is unlikely to follow it, making it, therefore, irrelevant for the current study.
Points were manually selected on the CTA scans for all patient datasets, and their assigned coordinates were based on the inherent coordinate system of each scan set (Fig. 1). Subsequently, using these 14 points and an in-house Matlab algorithm (version R2015b, MathWorks), 10 angles were calculated in space, as well as their projections on the frontal, the sagittal and the transverse planes (Figs. 2–4 and Table 2), hereafter mentioned as XZ, YZ and XY planes respectively. The advantage of this strategy lies at the ease of identifying the points of interest on the CTA scans. Rather than directly measuring the angles, we limited ourselves into identifying points, reducing the interpretation error. The same process is used in specialized, commercial software as well (e.g. EndoSize®, Therenva SAS, France), yet only for a couple of angles and only in 3D space.
The angles for all patients were calculated and the median and range of them documented. Note that in accordance to the literature, when calculating the angles in space, angles are always ≤180°. The same is not true, though, for the projected angles. The projections of the angles on the XZ, YZ and XY planes may be >180°; Figs. 2–4 help define their positive direction.
As a consequence of the analysis, a patient’s aortic shape can be concisely reported as a 1 × 10 matrix Φ:
using 3D space angle values, for the clinical practice. Similarly, a 3 × 10 matrix that reports the projection of these angles in 3 planes could be used to describe the aortic tree shape for computer-aided design (CAD) modelling in bioengineering applications:
Finally, a sensitivity analysis regarding the impact of small variations in the choice of the 14 critical points on the angles was performed. With the use of a Matlab algorithm, all coordinates were randomly translated by up to 1 mm in space and the effect on the angles was measured. The process was repeated 100 times, to simulate the inter-variance of measurements taken by different users.
Aortic dimensions
Regarding AAA dimensions, 9 variables were considered per patient (Table 3 and Fig. 5). Opposite to the previous section, these measurements were conducted by trained technicians of M2S, as part of the initial clinical investigation. Nevertheless, to address any possible limitations of the study, several CTA scans were examined by the authors as well, verifying that the dimensional measurements provided could be accurately reproduced.
All diameters were measured on CTA slices orthogonal to the centreline of the vessel and were calculated to the intima. Diameters have an accuracy of ±1 mm apart from the maximum one (±1.5 mm). The neck length has an accuracy of ±1.5 mm and the renal to Bi length ±2.5 mm. Finally, the volume is expressed in a 5% maximum range error. It should also be noted that 9 tortuosity values were missing due to limitations in the CTA scan images (either the right or left femoral bifurcation was not included in the scans); these values were treated as missing during the statistical analysis.
Statistical analysis
The collected data were analysed using SPSS (version 25). Normality was tested according to the Shapiro-Wilk test at the 0.05 significance level. Correlation between the examined variables was investigated with a 2-tailed Pearson correlation test at the 0.01 significance level. Lastly, the difference of medians between the genders, as well as symmetry propositions, were examined with the non-parametric, median test for 2 independent medians at the 0.05 significance level.
Results
Aortic angles
The median, the interquartile range (IQR) and range of all angles in their 3D and 2D manifestations are reported in Tables 4 and 5. With the exception of a few variables (φ1, φ2, φ4, φ8, in 3D space and φ4 in YZ-plane as well as φ6 in XY-plane), no angles were found to follow the normal distribution. The same was true for the dimensional variables as well (Table 6). It was this observation that lead to the decision to study the medians instead of the means of each variable for a more representative quantification, since medians are resistant to outliers. Figure 6 provides boxplots of all the angles, illustrating the significantly greater range of values for the projected angles compared to the 3D ones.
Lastly, the sensitivity analysis in the choice of the 14 critical points demonstrated small angle alterations, with the median discrepancy being below 0.3% for all angles (Table 7). The average minimum difference of all angles was just 0.01%, while the average maximum was 11.40%. Generally, the discrepancy was higher in the angles φ1and φ2 as these angles are calculated via points that are close to each other, hence offset variations of those coordinates result in larger angle alterations.
Aortic dimensions
The median, the IQR and range of all dimensional variables examined is reported in Table 8. Note that the iliacs are required to have a minimum diameter of usually 7 mm for the delivery of the endograft2, a requirement that herein was fulfilled since all of the examined patients underwent EVAR.
Statistical analysis
The first proposition to be tested was the lateral and anterior-posterior symmetry of the AAA. In general, AAAs appear to be significantly asymmetric and herein, the medians of the pairs of angles φ1– φ2, φ5– φ8, φ6– φ9 and φ7 –φ10 were compared. The analysis showed that only φ7–φ10 in 3D space, and φ1– φ2 and φ5– φ8 on the XZ-plane have statistically insignificant differences. For all other angles and angle projections, the left side of the AAA was different to the right one. Particularly for φ3 and φ4 on the XZ-plane, medians were <180°, creating a slight, general trend of neck angulation to the right. Regarding the YZ-plane, φ4 had a median of 224°, showing a greater anterior expansion, similar to φ5 (225°).
A few angles appeared to have weak linear correlations with coefficients ranging between 0.1 and 0.3. The only pair with a moderate correlation though, was φ1–φ2, with a correlation value of 0.542, suggesting that when the angle of one renal increases, the angle of the other will have a tendency to increase as well.
Subsequently, a series of correlations were tested for the dimensional variables of the study. Moderate correlations emerged between the pairs Volume – Average Tortuosity Index (coefficient ρ = 0.299), Age – Average Tortuosity Index (coefficient ρ = 0.399), Volume – Aneurysmal Length (coefficient ρ = 0.590) and Right Iliac Landing Diameter – Left Iliac Landing Diameter (coefficient ρ = 0.612). Finally, the variables Volume – Maximum Diameter were strongly correlated (coefficient ρ = 0.814).
The effect of gender was also examined. In the literature, body size information (i.e. height and weight) have been reported to influence and explain reasons for gender differences and are often employed in such comparisons16,17. Nevertheless, because such data were not available herein, all dimensional variables were indexed to (i.e. divided by) Average Neck Diameter to acquire normalized data. Via this process, it was identified that the normalized maximum diameter of the aneurysm was statistically significantly bigger in males as well as the normalized Renal to Bi length and the normalized Average Tortuosity Index (Table 9). It is interesting to note that in a sensitivity analysis, during which the measurement data were altered inside their error margin, the normalized maximum diameter was not always statistically significantly different for the two genders, yet the difference in the other two variables remained. Regarding the angles in 3D space, φ2 appeared statistically significantly different between the genders (99° vs 88° for males and females respectively), similarly to φ4 (119° vs 109°) and φ6 (132° vs 145°) (Table 10).
Histograms of all the (non-normalized) variables are provided in the Supplementary Information.
Discussion
Despite the fact that the geometrical complexity of the aneurysmal site is highly influenced by the angles of the aorta, the renal arteries and the iliacs, documentation of them is extremely rare in the literature. In this retrospective study, the aim was to identify all major angles involved in an AAA, to allow the description and reproduction of its shape. At the same time, common dimensional measurements were recorder and statistically analysed. The data used were obtained from a clinical study of the AnacondaTM endograft. This device is considered to be able to tackle severe angulations and is used for challenging EVAR cases18, hence choosing to acquire data from the specific clinical trial was regarded appropriate, in order to examine a wide range of geometries.
The angle set being proposed herein is a major extension to the angles commonly used. Both in the literature and specialized clinical software, attention is given to the neck angles alone, herein called φ3 and φ4. By mapping the entire AAA region though, a unique insight into the pathology has been achieved. The extreme values identified (see for example the very low values of φ4 in 3D space or of φ4 and φ8 in the XZ-plane) could be used by stent graft manufacturers to build (experimental or numerical) AAA case studies that would allow them to identify the operating range of their endografts. Moreover, the lack of strong correlation between aortic angles suggest that an investigator is free to construct any extreme AAA case-study with the values provided in Tables 4–5 in a mix-and-match manner. This strategy can have a powerful effect in EVAR stent graft designing. Looking further into the future, surgeons could eventually perform measurements on challenging patient CTA scans and decide which EVAR device could tackle best a specific geometry according to the Instructions for Use of each manufacturer.
It is important to acknowledge that from a clinical perspective, not all variables are equally significant for EVAR. Angles φ1 and φ2 relate to the renals and become relevant only when the aneurysmal neck is too short and a fenestrated endograft needs to be used. On the contrary, angles φ3 and φ4 are always important since greater neck angulation increases endoleak flow19. Li et al.20 suggested that when the aortic neck (herein φ3) is lower than 145°, devices with high fixation forces should be used. They also mention that the anterior-posterior neck angle (φ3 on the YZ-plane) influences the migration force of the stent graft significantly. The lower angles studied (i.e angles φ5 – φ10) refer to the iliac arteries and are critical for the successful delivery of the endograft. If too acute, open aortic surgery should be preferred.
It should be highlighted that the CTA scans obtained for this study reflect a specific group of AAA patients, i.e. those who undergo EVAR. As a result, the conclusions drawn should be generalized to the overall AAA population with caution. Ideally, an equivalent set of angle values corresponding to the healthy population should also be pursued. Such a set would serve as reference for the reported results. Moreover, it would allow a more holistic view of the pathology and its effect on the aorta. In future, a comparison between the healthy and the aneurysmal aortic shape should be conducted and possibly shed light into the development of the disease.
In this study, no dimensional and almost no angular variables followed a normal distribution. Frank et al.15 reported a similar finding when examining the maximum AAA diameter. In agreement to the results presented here, Wolf et al.5 have also reported a positive correlation of age and tortuosity and an absence of connection between age and size of the aneurysmal sac. In their study, though, the size of the aneurysm was also unrelated to tortuosity, a result that was not confirmed herein. A similar disagreement is raised with results of Bayle et al.21 who reported that the bigger the diameter of the aneurysm, the shorter the aneurysmal neck length becomes. No such correlation was observed herein, yet the results of Bayle et al. were acquired from the general AAA population. It is also worth noting that both these studies had significantly less patients enrolled (75 and 86 respectively). Finally, the asymmetry of the AAA has been mentioned by other investigators as well20.
In the attempt to identify differences in the shape of the AAA between the two genders, 3 angles were found to have statistically significant different medians. It is possible, however, that the number of these angles might be an overestimation because of the multiple post hoc comparative tests, and that similarities are even greater between the two genders.
AAA incidents are four to six times more frequent in men than in women22. In the present study, the number of male patients was 6.5 times greater than the number of females, implying that males might have been slightly overrepresented. Nevertheless, the framework built for the angular characterization of the aneurysmal aorta, along with the demonstration that the AAA angles do not, in general, correlate with each other, can bring new insight to the topic. The process developed has allowed the establishment of representative and worst case geometries of AAAs that can be an aid to successful EVAR. The data produced herein can be used to create CAD aortic models for virtual deployment, testing and optimization of endografts during their development phase, or assist the characterization of patient specific AAAs (e.g. as extreme, treatable, or treatable with a specific stent graft). To the authors’ knowledge, this is the most extensive geometrical study of the AAA shape.
Data availability
All datasets associated with this manuscript are available in FIGSHARE (https://doi.org/10.6084/m9.figshare.9897632) and from the corresponding author upon reasonable request.
References
Sethi, R. K. V. et al. Impact of hospital market competition on endovascular aneurysm repair adoption and outcomes. J. Vasc. Surg. 58, 596–606 (2013).
Walker, T. G. et al. Clinical Practice Guidelines for Endovascular Abdominal Aortic Aneurysm Repair: Written by the Standards of Practice Committee for the Society of Interventional Radiology and Endorsed by the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol 21, 1632–1655 (2010).
Ahn, S. S. et al. Reporting standards for infrarenal endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 25, 405–410 (1997).
Chaikof, E. L. et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J. Vasc. Surg. 35, 1061–1066 (2002).
Wolf, Y. G. et al. Impact of aortoiliac tortuosity on endovascular repair of abdominal aortic aneurysms: Evaluation of 3D computer-based assessment. 594–599 https://doi.org/10.1067/mva.2001.118586.
Carpenter, J. P. et al. Impact of exclusion criteria on patient selection for endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 34, 1050–1054 (2001).
Wolf, Y. G. et al. Endovascular repair of abdominal aortic aneurysms: Eligibility rate and impact on the rate of open repair. J. Vasc. Surg. 32, 519–523 (2000).
Armon, M. P. et al. Anatomical suitability of abdominal aortic aneurysms for endovascular repair. Br. J. Surg. 84, 178–180 (1997).
Kristmundsson, T. A Novel Method to Estimate Iliac Tortuosity in Evaluating EVAR Access. 157–164 (2012).
Henretta, J. P. et al. Special iliac artery considerations during aneurysm endografting. Am. J. Surg. 178, 212–218 (1999).
Clough, R., Hertault, A., Azzaoui, R., Sobocinski, J. & Haulon, S. Low-Profile EVAR. Endovasc. today 15, 72–75 (2016).
Li, Z. & Kleinstreuer, C. Analysis of biomechanical factors affecting stent-graft migration in an abdominal aortic aneurysm model. J. Biomech. 39, 2264–2273 (2006).
Morris, L., Delassus, P., Walsh, M. & McGloughlin, T. A mathematical model to predict the in vivo pulsatile drag forces acting on bifurcated stent grafts used in endovascular treatment of abdominal aortic aneurysms (AAA). J. Biomech. 37, 1087–95 (2004).
U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/home.
Frank A. L., et al. Variability in measurement of abdominal aortic aneurysms. J. Vasc. Surg. 945–952 (1995).
Jones, G. T. et al. Correcting for Body Surface Area Identifies the True Prevalence of Abdominal Aortic Aneurysm in Screened Women. Eur. J. Vasc. Endovasc. Surg. 57, 221–228 (2019).
Matyal, R. et al. Impact of gender and body surface area on outcome after abdominal aortic aneurysm repair. Am. J. Surg. 209, 315–323 (2015).
Rödel, S. G. J., Zeebregts, C. J., Huisman, A. B. & Geelkerken, R. H. Results of the Anaconda endovascular graft in abdominal aortic aneurysm with a severe angulated infrarenal neck. J. Vasc. Surg. 59, 1495–1501.e1 (2014).
Albertini, J.-N., Macierewicz, J. A., Yusuf, S. W., Wenham, P. W. & Hopkinson, B. R. Pathophysiology of Proximal Perigraft Endoleak Following Endovascular Repair of Abdominal Aortic Aneurysms: a Study Using a Flow Model. Eur. J. Vasc. Endovasc. Surg. 22, 53–56 (2001).
Li, Z., Kleinstreuer, C. & Farber, M. Computational analysis of biomechanical contributors to possible endovascular graft failure. Biomech. Model. Mechanobiol. 4, 221–34 (2005).
Bayle, O. et al. Morphologic assessment of abdominal aortic aneurysms by spiral computed tomographic scanning. J. Vasc. Surg. 26, 238–246 (1997).
Starr, J. E. & Halpern, V. Abdominal aortic aneurysms in women. J. Vasc. Surg. 57, 3S–10S (2013).
Acknowledgements
The medical data used were kindly provided by M2S, Inc. West Lebanon, NH, USA and Terumo Aortic. The authors gratefully acknowledge the financial support provided by Medical Research Scotland, project PhD-723–2013 and by Terumo Aortic for scholarship funding, as well as EPSRC (grant ref.: EP/R51178X/1) for further financial support.
Author information
Authors and Affiliations
Contributions
F.K. design of the study, data acquisition and analysis, F.K., W.D., D.N. manuscript preparation and critical manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kyriakou, F., Dempster, W. & Nash, D. A Methodology to Quantify the Geometrical Complexity of the Abdominal Aortic Aneurysm. Sci Rep 9, 17379 (2019). https://doi.org/10.1038/s41598-019-53820-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53820-z
- Springer Nature Limited
This article is cited by
-
Iliac Artery Tortuosity, Calcification and Abnormal Shape Augment Aortic Aneurysm Anatomy in Predicting Complications After Endovascular Aneurysm Repair
SN Comprehensive Clinical Medicine (2022)