Abstract
We investigated covariates of pharmacokinetics of micafungin in critically ill patients. After application of micafungin, plasma samples were collected. Non-linear mixed effects modelling (NONMEM 7.3) was used to develop the pharmacokinetic model. Using this model, the adequacy of a fixed 100 mg dosing regimen was evaluated in the study cohort. A two-compartment model with linear elimination was found to describe the obtained data. SOFA score was identified as a significant covariate on both clearance and central volume of distribution, respectively. Patients in highly critical condition, represented by a SOFA above 10 showed a 30.8% lower central volume of distribution than the less critically ill patients. For patients with bilirubin levels above 4 mg/dl, clearance was decreased by 21.1%. Renal replacement therapy (RRT) did not influence micafungin clearance or the volumes of distribution. In a posthoc evaluation of the modeled population, 100 mg micafungin was suitable when assessing the PKPD targets (AUC/MIC) for C. albicans and C. glabrata, with insufficient target attainment for C. parapsilosis. Micafungin pharmacokinetics appear not to be influenced by the status of RRT. A dose of 100 mg micafungin is suitable for infections with C. albicans and C. glabrata in critically ill patients.
Similar content being viewed by others
Introduction
Invasive fungal infections in critically ill patients are a serious threat and a cause of high mortality. In unselected patient cohorts, fungi account for 10–20% of infections in the intensive care unit. Candida species are the predominant fungi isolated in critically ill surgical patients1,2. A typical focus of infection in this collective is the intraabdominal space, where Candida peritonitis and candidemia develop after perforation of a hollow viscus3. Besides this typical mechanism, candidemia can also occur as a device-related infection, e.g. by inoculation through an intravascular catheter4,5.
The contemporary treatment of invasive candidiasis and candidemia relies on echinocandines as the primary choice of antifungal agents. This strategy is recommended by several clinical guidelines dealing with invasive fungal infections6,7,8. After caspofungin and anidulafungin, micafungin was the third echinocandin antifungal agent approved by the European Medicines Agency in 2008. It is licensed for the treatment of all types of invasive candidiasis, esophageal candidiasis and for prophylaxis in patients undergoing stem cell transplantation9.
Micafungin is available as an intravenous formulation, which is typically infused over 1 h. It is characterized by potent antifungal activity, with few drug interactions and no relevant toxicity. The mechanism of action of micafungin is the inhibition of fungal β-(1, 3) glucan synthase complex, leading to the depletion of cell-wall glucan and consecutive osmotic instability causing cell death9.
Echinocandins exhibit concentration-dependent killing of Candida species. Therefore, the 24-h area under the concentration time curve divided by the minimal inhibitory concentration (AUC/MIC) is the pharmacodynamic parameter that best describes the dose-response relation of this drug10. Micafungin has a small volume of distribution (~119 ml/kg) and the drug is highly protein bound (>99%). The primary binding protein is albumin. Linear pharmacokinetics were observed for daily doses between 12.5 mg and 200 mg as well as 3 mg/kg and 8 mg/kg9. The pharmacokinetics and pharmacodynamics of micafungin have recently been described by Wasmann et al. in detail11.
Regarding special populations, Herbert et al. did not find an effect of altered renal dysfunction on micafungin pharmacokinetics in a non-compartmental analysis12. In patients with hepatic dysfunction, micafungin AUC was lower than in control subjects, other pharmacokinetics parameters such as weight adjusted clearance or volume of distribution did not show a statistical difference12. According to Undre et al., AUC is significantly lower, and clearance was found to be higher in patients with severe hepatic dysfunction, which might be secondary to reduced plasma albumin levels in these subjects13. An increase in free drug levels during hypoalbuminemia is thought to cause an increased clearance in these patients.
No dose adjustment is necessary for elderly patients, as exposure and disposition of micafungin in healthy subjects aged 66–78 years were not significantly different from those aged 20–24 years9.
Previous pharmacokinetic analyses indicate a body weight-dependent clearance of micafungin. There was no relationship between weight and clearance in subjects between 43 kg and 66 kg, but the clearance increased with a function of weight, beyond a threshold of 66 kg. It remains to be determined, whether this finding suggests that subject weighing more than 66 kg might fail to achieve optimal AUC/MIC ratios with standard dosages14,15.
Critically ill patients in the intensive care unit (ICU) are a heterogeneous cohort of patients. Various degrees of organ dysfunction, changes in volume status and metabolic imbalances signify critical illness. Taken together, these physiologic abnormalities have considerable influences on the pharmacology of many substances. Regarding antiinfectives, these changes can have deleterious consequences. If the exposure to an antibiotic or antimycotic compound is too low, the efficacy is compromised, subsequently raising the potential of therapeutic failure16.
The objective of this pharmacokinetic study was to assess the pharmacokinetics of micafungin in a mixed cohort of critically ill adult patients with diverse organ failures, including renal failure supported by renal replacement therapy (RRT). Furthermore, the impact of different modes of RRT on pharmacokinetics was explored. Blood levels were then correlated with AUC/MIC of different candida species to evaluate pharmacologic adequacy of a daily dose of 100 mg micafungin.
Results
This study of micafungin pharmacokinetics included 36 patients (24 male and 12 female) aged between 22 and 84 years. The median weight was 94.5 kg (49.4 kg–162 kg).
All patients included were critically ill, as reflected by high compound scores for critical illness (APACHE 2, SOFA, SAPS 2). The majority of patients fulfilled sepsis criteria (SEPSIS-II), received vasopressors for hemodynamic support and were on mechanical ventilation. 23 patients received renal replacement therapy (RRT) for at least one day of the study while the remaining 13 patients never received RRT. A summary of patient characteristics and covariates is shown in Table 1.
Micafungin was started empirically in 25 patients, where the suspected foci of infection were either intraabdominally or device-associated (intravenous catheters, ECMO). In 11 patients the prescription was made to treat proven infections. Of those 11 patients, 8 had intraabdominal infections with candida species in intraoperative samples, two had candidemia with suspected infection of venous catheters and one had osteomyelitis of the sternum and mediastinitis after heart surgery. Candida albicans was isolated in 8 cases, Candida glabrata in 3 cases. The median length of micafungin therapy was 10,5 days (ranges 2–79 days) and treatment was continued for as long as treating physicians felt it was adequate. 15 patients died in the ICU; the other patients were discharged alive.
The measured plasma concentration plotted against time after first dose or time after last dose are shown in Figs 1 and 2, respectively. Samples were available at various time points across the treatment period, giving the opportunity to study micafungin pharmacokinetics during up to 10 days of treatment. No accumulation of micafungin was observed and a marked heterogeneity of drug concentrations in the critically ill patient population is clearly visible (Figs 1, 2).
Population pharmacokinetic modelling
The basic structural model consisted of two compartments with a proportional error model. Clearance was tested as linear, nonlinear Michaelis-Menten type and as combined linear- nonlinear type. Out of these, the linear clearance best described the data. Interindividual variability (IIV) was allowed for clearance, central and peripheral volume of distribution. For these variabilities, a full covariance matrix was estimated.
Covariate testing was performed using stepwise covariate modelling (SCM) in PsN with a 5% forward inclusion and 1% backward elimination criterion. Through SCM, a significant covariate effect was shown for the SOFA score with a linear decrease in clearance with a factor of 0.024 per score point.
Plots of the parameters against covariates showed additional relations for SOFA on central volume of distribution, calculated as categorical effect for critically ill patients with a SOFA >10, resulting in a 30.8% lower central volume of distribution in the critically ill group.
In addition, a categorical effect for bilirubin on clearance with a cut-off for bilirubin >4 mg/dl was included, showing a 21.1% lower clearance in the group with elevated bilirubin levels. Parameter estimates for the final model are listed in Table 2.
All other covariates did not show a significant influence on any of the PK parameters. An influence of the patients’ body weight, especially with obese patients, as reported by Hall et al.15 was not detected. Renal replacement therapy modalities (CVVHDF, SLEDD) were correlated with SOFA scores, indicating that highly critically ill patients tend to have a higher chance of receiving renal replacement therapy at any time during treatment and more CVVHDF treatments are recorded in this group. The visual exploration of the differences in SOFA distribution for distinct modalities of RRT is shown in Fig. 3. During the study, patients could switch between the modalities as clinically necessary and by decision of the treating physicians. This somewhat random shift makes a classical correlation analysis difficult. A plain analysis of differences of the groups’ mean SOFA scores by t-test showed differences (p > 0.01) between mean SOFA scores across all treatment groups (no RRT, CVVHDF, SLEDD). The impact of disease state (i.e. critical illness reflected by SOFA score) and RRT group were tested independently on the estimated population pharmacokinetic parameters, revealing only an influence of the SOFA score as a relevant covariate.
Internal model validation performed by GOF (goodness of fit) plots showed an even distribution of residuals over TAD (time after dose) and PRED (population model predictions) (Fig. 4). A VPC (visual predictive check) was created against time after dose and against the SOFA score to investigate the appropriateness of the model over the whole time and SOFA range, as shown in Figs 5, 6. In this VPC, the observed percentiles and median matched the corresponding simulated confidence intervals, indicating an adequate predictive performance of the final model.
Pharmacodynamic target evaluation
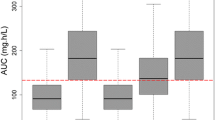
In a post-hoc estimation step, the AUC was determined for each patient on each day of treatment. The resulting AUC/MIC ratios for different candida species are shown in Figs 7–9. When compared to common Candida species breakpoints, the AUC/MIC ratios show adequate therapy intensities for both C. albicans (Fig. 7) and C. glabrata (Fig. 8), whereas a fixed dose of 100 mg micafungin does not seem suitable for the less sensitive C. parapsilosis strains (Fig. 9).
AUC/MIC ratio for micafungin and C. albicans in the observed study population; Each box represents 24 h of therapy, numbers below boxes show the number of patients enrolled in the study at the respective time point, solid line represents the EUCAST cut off value for antifungal activity for C. albicans.
AUC/MIC ratio for micafungin and C. glabrata in the observed study population; Each box represents 24 h of therapy, numbers below boxes show the number of patients enrolled in the study at the respective time point, solid line represents the EUCAST cut off value for antifungal activity for C. glabrata.
AUC/MIC ratio for micafungin and C. parapsilosis in the observed study population; Each box represents 24 h of therapy, numbers below boxes show the number of patients enrolled in the study at the respective time point, solid line represents the EUCAST cut off value for antifungal activity for C. parapsilosis.
Discussion
Since micafungin was licensed for clinical use in 2008, various parametric and non-parametric PopPK models have been published. In accordance with our findings, they all consist of two compartments with linear elimination12,15,17,18,19.
The reported PopPk model well describes the pharmacokinetics of micafungin in this critically ill population. The main objective of this study was to further investigate the influence of different types of renal replacement therapy on micafungin kinetics. In our analysis, renal replacement therapy did not alter the pharmacokinetics of micafungin. Neither continuous dialysis (CVVHDF), nor intermittent modes of dialysis (SLEDD) caused appreciable removal of micafungin. This finding is in accordance with the known elimination routes of micafungin and adds to the recently published observations of Vossen et al.20.
Rather than the status of kidney failure or renal replacement therapy, the overall severity of illness as reflected by the SOFA score has an influence on elimination and distribution of micafungin. In patients suffering from liver dysfunction with bilirubin levels above 4 mg/dl as part of multi organ dysfunction, Micafungin clearance decreased. This finding is in accordance with the biliary excretion of micafungin, which will be affected in situations of liver dysfunction.
Taken together, the results of our analysis are in keeping with other published findings examining pharmacokinetics of micafungin in critically ill patients17,19,21,22. However, the covariate model differs slightly from previous findings.
Martial et al.19 tested, but could not confirm an influence by the covariates albumin, Child Pugh Score, SOFA Score or renal replacement therapy in a parametric PopPk analysis. Jullien et al.17 reported a categorical influence of the SOFA score as well as albumin levels on the micafungin clearance, explored by a parametric PopPk analysis. These dissimilar findings might be explained by differences in the time points of blood sampling. In our study, patient samples were collected for up to seven days, if therapy with micafungin was continued. This allowed a valid analysis of time-dependency of different factors and their influence on pharmacokinetics, that have not been covered in previous analyses.
In this cohort of critically ill patients with suspected or proven infection with Candida ssp., once daily dosage of 100 mg micafungin resulted in adequate plasma levels of the drug in all patients, when assuming the EUCAST-breakpoints for C.albicans and C. glabrata (Figs 7, 8). In infections with C. parapsilosis, our analysis revealed inadequate exposure with once daily dosing due to higher breakpoints (Fig. 9). This finding corroborates previous reports of questionable efficacy of micafungin in this particular fungus23. We conclude that alternative substances might be a preferable choice, for example azole antifungals or preparations of Amphotericin B.
As the EUCAST-breakpoints are slightly lower than their CLSI counterparts, our analysis differs from other studies that aim to attain numerically higher pharmacokinetic targets21,22. As the clinical relevance of this difference in targets is unknown, we feel that a widespread use of increased doses for non-parapsilosis Candida species is not warranted at the moment22.
Nevertheless, the recommended dosing regimen of once daily application of 100 mg micafungin can be considered adequate for the majority of critically ill patients with suspected or proven candidiasis. Renal replacement therapy does not have an influence on the disposition of micafungin and changes in dosing are not warranted in this collective. Therefore, our study adds to the body of evidence demonstrating the adequacy of a comparatively easy dosing scheme for micafungin without the necessity of dynamic adjustments in a wide range of patients with critical illness. However, if infections with C. parapsilosis are detected, target attainment is more difficult than in cases of infection with C. albicans or C. glabrata. If a switch to alternative substances is not feasible in these cases, micafungin therapy would benefit from therapeutic drug monitoring and resistance testing in order to reach sufficient PK/PD-targets.
Methods
Study protocol
This is a non-interventional study conducted in critically ill adult patients treated in the intensive care units of the Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy of the University Hospital Muenster in Germany. The collection of data and blood samples was approved by the ethics committee of the University of Muenster (Study Code 06-AnIt-11). All procedures performed in in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients or their representatives.
The study included 36 adult patients. Patient demographics and other clinical parameters were collected from the electronic patient charts of the intensive care unit. All patients received 100 mg micafungin intravenously once daily either as empiric or targeted therapy of suspected or proven infection. The decision to begin or end therapy was made by the physicians in charge of the intensive care units irrespective of the pharmacologic study.
Blood samples were collected from indwelling arterial catheters for analysis of micafungin concentrations. On day 1, plasma micafungin concentration was determined at 1, 4, 8, 12, 16 and 20 hours post application and as a trough level immediately before the next dose of micafungin after 24 hours. From day two of therapy, peak levels one hour after infusion and trough levels were collected.
Blood sampling
Blood samples were exclusively taken from arterial lines into ethylenediaminetetraacetic acid (EDTA)-containing sample vials tubes (S-Monovette® 2.7 ml K3E, Sarstedt, Nümbrecht, Germany). Samples were immediately transported to the in-house laboratory and plasma was separated by centrifugation at 1600 x g (10 min) and kept and frozen at −80 °C until analysis.
Sample preparation and analysis of micafungin
Micafungin in human plasma was analyzed with reversed-phase high-performance liquid chromatography. 200 µl serum, 20 µl internal standard solution (anidulafungin 1 mg/ml in water), 20 µl 30% ZnSO4 x7H2O (w/v) in water and 700 µl methanol were vortex-mixed (5 sec) followed by centrifugation at 800 g for 3 min. 50 µl of the supernatant was injected on a 125 liquid chromatograph (Beckman Coulter GmbH, Krefeld, Germany) interfaced with a model 168 diode array detector. Chromatographic conditions: mobile phase: acetonitrile/50 mM ammonium acetate buffer pH 4.6, 50:50 (v/v); flow rate 1 ml/min at 45 °C; absorbance detection 271 nm; column: XTerra RP18, 3.5 µm, 150 × 4.6 mm (Waters GmbH, Eschborn, Germany).
The method was validated according to the guidelines for bioanalytical methods published by the European Medicines Agency24. The recoveries using this precipitation procedure were 99 ± 3.3% and 96.2 ± 5.8% for 5 and 200 µg/mL micafungin in serum, respectively. The lower limit of quantitation (LLOQ), the limit of detection (LOD) and the linear range for micafungin analysis were 0.1 µg/ml, 0.02 µg/ml, and 0.1–200 µg/ml, respectively. The intra- and inter-day precision of the assay expressed as a coefficient of variation (CV%) ranged from 3.7 to 7.2%.
Population pharmacokinetic modelling
Data pre-analysis and graphical output was created in R (version 3.4.0; R foundation for statistical computing, Vienna, Austria) with additional use of the Xpose package (version 4.5.3).
Model building was performed using NONMEM (Version 7.3; ICON Development Solutions, Ellicott City, MD) with the ADVAN 6 subroutine and the FOCE + I estimation method. Visual predictive checks (pcVPC) and stepwise covariate model building (SCM) were performed with the PsN module (Version 4.6.0)25. Graphical output was created in R (version 3.4.0; R foundation for statistical computing, Vienna, Austria) with additional use of the Xpose package (version 4.5.3)26.
The basic structural model was tested with the following compositions: One to three compartments with linear clearance, nonlinear (Michaelis Menten) clearance and a combination of both were tested.
There was no covariate inclusion before the basic structural model was defined.
For nested models, the difference in the objective function value (OFV) was considered the best parameter to quantify model improvement. A drop in OFV of 3.84 and 6.63, corresponding to a 5% and 1% level of significance respectively, was considered an adequate model improvement, when a single parameter was added. Non-hierarchical models were compared using the Akaike information criterion (AIC) in combination with goodness of fit (GOF) plots27,28.
All covariates were collected as time-varying covariates, if feasible.
Potential continuous covariates included demographics such as weight, age and BMI. Additionally, albumin and serum-protein were documented to depict changes in protein binding. Renal function was documented for non-RRT-patients by creatinine clearance (CrCl) and creatinine values. Hepatic function was characterized through documentation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), butyrylcholinesterase (BCHE) and bilirubin. As general scores, Acute Physiology and Chronic Health Evaluation II (APACHE II) was recorded on admission to the ICU and both Sepsis-related organ failure assessment score (SOFA) and Simplified Acute Physiology Score (SAPS2) scores were documented each day in the ICU.
As categorical covariate, sex was tested. Covariate influence was tested on central and peripheral volumes of distribution, and clearance. Covariate testing was performed using stepwise covariate modelling (SCM) in PsN with a 5% forward inclusion and 1% backward elimination criterion29.
Model performance was evaluated by creating GOF plots including individual predictions (IPRED) and population predictions (PRED) versus the observed values, and plots for conditional weighted residuals (CWRES) versus time, time after dose (TAD) and the population model predictions (PRED). Visual predictive checks (VPCs) were created to test the predictive performance of the model.
Pharmacodynamic target evaluation
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) established the guideline for micafungin and Candida ssp. with species related clinical breakpoints for non-resistant strains10. These breakpoints were used as surrogate for the minimal inhibitory concentration (MIC) for C. albicans, C. glabrata and C. parapsilosis. Proposed MICs are 0.016 g/L, 0.03 g/L and 2 g/L, respectively.
Additionally, EUCAST suggests the following AUC/MIC targets to reach stasis: C. albicans (2420), C. glabrata (1283), C. parapsilosis (285).
Using the previously established population pharmacokinetic model, the AUC for each patient on each day of therapy was determined. In a post-hoc estimation step, micafungin concentrations in the central compartment were used to interpolate for AUC 0-24 h on each day of treatment and the AUC/MIC ratio was calculated and compared to the guideline suggestions.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
SepNet-Critical-Care-Trials-Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med 42, 1980–1989, https://doi.org/10.1007/s00134-016-4504-3 (2016).
Engel, C. et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33, 606–618, https://doi.org/10.1007/s00134-006-0517-7 (2007).
Bassetti, M. et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med 41, 1601–1610, https://doi.org/10.1007/s00134-015-3866-2 (2015).
Bassetti, M. et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med 43, 1225–1238, https://doi.org/10.1007/s00134-017-4731-2 (2017).
Kullberg, B. J. & Arendrup, M. C. Invasive Candidiasis. N Engl J Med 373, 1445–1456, https://doi.org/10.1056/NEJMra1315399 (2015).
Cornely, O. A. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7), 19–37, https://doi.org/10.1111/1469-0691.12039 (2012).
Cuenca-Estrella, M. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 18(Suppl 7), 9–18, https://doi.org/10.1111/1469-0691.12038 (2012).
Pappas, P. G. et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62, e1–50, https://doi.org/10.1093/cid/civ933 (2016).
U.S. Food and Drug Administration. Mycamine (micafungin sodium) for injection. Product label U.S. Food and Drug Administration. Available at, http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021506s009lbl.pdf (2007).
Arendrup, M. C., Cuenca-Estrella, M., Lass-Florl, C. & Hope, W. W. & European Committee on Antimicrobial Susceptibility Testing - Subcommittee on Antifungal Susceptibility, T. EUCAST technical note on Candida and micafungin, anidulafungin and fluconazole. Mycoses 57, 377–379, https://doi.org/10.1111/myc.12170 (2014).
Wasmann, R. E. et al. Clinical Pharmacokinetics and Pharmacodynamics of Micafungin. Clin Pharmacokinet 57, 267–286, https://doi.org/10.1007/s40262-017-0578-5 (2018).
Hebert, M. F. et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol 45, 1145–1152, https://doi.org/10.1177/0091270005279580 (2005).
Undre, N., Pretorius, B. & Stevenson, P. Pharmacokinetics of micafungin in subjects with severe hepatic dysfunction. Eur J Drug Metab Pharmacokinet 40, 285–293, https://doi.org/10.1007/s13318-014-0204-y (2015).
Gumbo, T. et al. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis 60, 329–331, https://doi.org/10.1016/j.diagmicrobio.2007.09.018 (2008).
Hall, R. G., Swancutt, M. A. & Gumbo, T. Fractal geometry and the pharmacometrics of micafungin in overweight, obese, and extremely obese people. Antimicrob Agents Chemother 55, 5107–5112, https://doi.org/10.1128/AAC.05193-11 (2011).
Roberts, J. A. et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14, 498–509, https://doi.org/10.1016/S1473-3099(14)70036-2 (2014).
Jullien, V. et al. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J Antimicrob Chemother 72, 181–189, https://doi.org/10.1093/jac/dkw352 (2017).
Lempers, V. J. et al. Altered Micafungin Pharmacokinetics in Intensive Care Unit Patients. Antimicrob Agents Chemother 59, 4403–4409, https://doi.org/10.1128/AAC.00623-15 (2015).
Martial, L. C. et al. Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients. Clin Pharmacokinet 56, 1197–1206, https://doi.org/10.1007/s40262-017-0509-5 (2017).
Vossen, M. G. et al. Micafungin Plasma Levels Are Not Affected by Continuous Renal Replacement Therapy: Experience in Critically Ill Patients. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.02425-16 (2017).
Boonstra, J. M. et al. Pharmacokinetic Properties of Micafungin in Critically Ill Patients Diagnosed with Invasive Candidiasis. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.01398-17 (2017).
Maseda, E. et al. Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Crit Care 22, 94, https://doi.org/10.1186/s13054-018-2019-8 (2018).
Yang, Q. et al. Pharmacokinetic/pharmacodynamic adequacy of echinocandins against Candida spp. in intensive care unit patients and general patient populations. Int J Antimicrob Agents 47, 397–402, https://doi.org/10.1016/j.ijantimicag.2016.02.004 (2016).
European Medicines Agency. Guideline on Bioanalytical Method Validation. Available at, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (2011).
Lindbom, L., Pihlgren, P. & Jonsson, E. N. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79, 241–257, https://doi.org/10.1016/j.cmpb.2005.04.005 (2005).
Jonsson, E. N. & Karlsson, M. O. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58, 51–64 (1999).
Byon, W. et al. Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst Pharmacol 2, e51, https://doi.org/10.1038/psp.2013.26 (2013).
Mould, D. R. & Upton, R. N. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2, e38, https://doi.org/10.1038/psp.2013.14 (2013).
Jonsson, E. N. & Karlsson, M. O. Automated covariate model building within NONMEM. Pharm Res 15, 1463–1468, https://doi.org/10.1023/a:1011970125687 (1998).
Author information
Authors and Affiliations
Contributions
S.G. collected data, performed pharmacokinetic analyses and wrote the manuscript. C.L. conceived the study, recruited patients, collected blood samples, collected data and wrote the manuscript. M.B. collected data. D.H. conceived the study, collected data, performed micafungin measurements and wrote the manuscript. M.F. conceived the study, collected data and performed micafungin measurements. D.B. collected blood samples and data. G.H. conceived the study, performed pharmacokinetic analyses and wrote the manuscript. B.E. conceived the study, recruited patients, collected blood samples and wrote the manuscript. All authors have reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
This study was supported with a research grant by Astellas Pharma to B.E. The funding source was not involved in the conception of the study, data collection, data analysis, decision to publish or the writing of the manuscript. C.L., D.H. and B.E. have received honoraria by Astellas Pharma for speaking at symposia. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gastine, S., Lanckohr, C., Blessou, M. et al. Pharmacokinetics of Micafungin in Critically Ill Patients. Sci Rep 9, 17741 (2019). https://doi.org/10.1038/s41598-019-53093-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53093-6
- Springer Nature Limited