Abstract
Costs and benefits of group living are a fundamental topic in behavioural ecology. Resource availability affects individuals’ breeding prospects alone and in groups, as well as how reproduction is distributed within groups (“reproductive skew”). Here, in facultatively social thrips, we provide correlational evidence that breeding resources are associated with (1) whether solitary or social living is favoured, and (2) the degree of ovarian skew. Dunatothrips aneurae (Thysanoptera, Phlaeothripidae) cooperatively build silk “domiciles” on Australian Acacias, feeding exclusively from internal phyllode surfaces. Per capita productivity scaled differently with group size depending on domicile volume — females in small domiciles did better alone than in groups, whereas in large domiciles single and group-nesting females did equally well. Ovarian dissections revealed that in small domiciles some females were nonreproductive, indicating ovarian (i.e. reproductive) skew. Skew increased as domicile size decreased and group size increased. Breeders had smaller oocyte volume in smaller domiciles, especially those containing nonreproductives. These findings suggest group formation and reproductive skew in D. aneurae may be influenced by reproductive competition for breeding resources. Nonreproductive females in small domiciles may be reproductively suppressed, subfertile, or accumulating resources to reproduce.
Similar content being viewed by others
Introduction
Is it better for animals to breed alone, or in groups? The size of animal breeding groups represents the balance of multiple benefits and costs to individuals1. From the perspective of costs, groups may form because costs of independent breeding outweigh costs of grouping2; such “ecological constraints” include, for example, habitat saturation in vertebrates, or nest construction in invertebrates3,4,5,6. Viewed the other way, the benefits to the individual of being in a group may outweigh the benefits of independent breeding7; for example, groups may be better than individuals at securing high-quality resources8. In general, breeding success depends upon access to a sufficient amount and quality of resources — whether individually9 or within groups10. We would therefore expect the size and quality of resources available to individuals inside and outside of groups to affect the relative costs and benefits of group breeding11,12.
Within a group, though, an individual’s breeding prospects are often far from guaranteed. Reproduction is often shared unequally — to an extent that is termed the reproductive skew13. While low-skew societies are relatively egalitarian, in societies with high skew, fully reproductive breeders exist alongside subordinate individuals that breed to a lesser extent or not at all, who may or may not assist breeders14,15,16. Competition for resources within groups may affect reproductive skew10,17. Thus, we might expect resource size and quality to be associated not only with group size, but also with reproductive skew.
Intuitively, we would expect groups inhabiting bigger or better resource patches to have lower skew; for any given group size, a better resource patch can support a higher proportion of individuals reproducing. In addition, better resource patches can also support larger groups — which may have lower skew simply by virtue of being larger groups, irrespective of resources, for three reasons. First, in larger groups it may be harder for a dominant individual to control breeding17. Second, the positive influence a single subordinate can have on the reproduction of a large group is smaller than when it is part of a small group. Thus, the incentive for it to leave the larger group and try to breed alone is greater, and it may therefore demand a greater share of reproduction from the dominant as a “staying incentive” for it to stay in the larger group18. Finally, in larger groups there may be a greater threat of aggressive challenges for dominance, such that dominant individuals may be more motivated to concede reproduction to subordinates as a “peace incentive”19,20.
Two lineages of Australian Acacia thrips (Thysanoptera, Phlaeothripinae) exhibit social behaviour. As haplodiploids, Acacia thrips present contrasts and comparisons with the better-studied social Hymenoptera, and are increasingly appreciated as a parallel model clade for social research21. Both of the social thrips lineages are herbivorous, the group members living, feeding and reproducing entirely within their resource. In the first lineage, the gall-inducing thrips, which are eusocial, a general evolutionary trend towards smaller galls (i.e. fewer resources) has been accompanied by more pronounced division of reproduction between foundress and soldiers (i.e. increasing reproductive skew)22,23,24. This finding was interpreted as evidence that within-gall competition for resources may have helped to drive the evolution of the soldier caste22. Yet, without a truly solitary option available, the effects of resource size upon the costs and benefits of social behaviour are hard to assess.
The other thrips lineage in which sociality has evolved contains several origins of “phyllode-glueing”, a much less well-studied lifestyle. Phyllode-gluers live and breed entirely within “domiciles”, which are nests constructed by cementing together Acacia phyllodes with a silk-like substance extruded from their abdomen21. Among these, a few species in the genus Dunatothrips show facultative pleometrosis, i.e. joint nesting. Females of some species are solitary (e.g. D. gloius), but in others can be found in groups of up to 4 in D. skene25, 8 in D. armatus21, ~15–20 in D. aneurae25,26,27 and >70 in D. vestitor21.
By far the best studied of these species is D. aneurae. In this species, reproductive females (“foundresses”) can be easily identified since they become dealate (lose wings) upon building a domicile, or occasionally upon entering an existing domicile, following dispersal27. Single foundresses comprise roughly 70% of the population25,26,28, showing that independent nesting is certainly feasible, and suggesting that ecological constraints driving social behaviour are weak. Furthermore, foundress numbers were found not to be correlated with domicile density at the tree level, suggesting that habitats are not locally saturated26. Nevertheless, per capita productivity appears to decline with increasing foundress numbers26, suggesting a cost of grouping.
Several possible benefits of joint nesting may counterbalance this cost. Cofoundresses tend to be relatives, enhancing inclusive fitness of group members28. Cofounding enhances defence against kleptoparasites26 although not against inquilines29, and also increases adult survival via other unknown means26 — which are hypothesized to involve sharing of costs associated with e.g. domicile building or maintenance21,27,30.
Variation in resources available to single versus multiple females may affect any of these costs and benefits — and ultimately whether social behaviour is favoured. Indeed, Crespi et al.21 proposed the hypothesis that resource variability may be one key reason why D. aneurae and D. vestitor may have evolved to have such a high degree of social flexibility. Domicile sizes in these species are highly variable because of the loose, multi-phyllode conformation of their domiciles compared to more solitary species, which tend simply to make a domicile in the diamond-shaped space created by two crossed phyllodes. In this study, we aim to test this hypothesis by asking whether the size of D. aneurae domiciles, i.e. of breeding resources (feeding area or breeding space), is associated with per capita reproduction and its distribution within a domicile (reproductive skew) and hence whether social or solitary behaviour is broadly favoured.
Results
Productivity
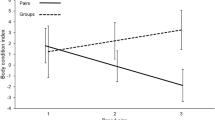
Domicile volume ranged from 7.75 mm3 to 1932.78 mm3 (mean 205.76 ± SE 11.35 [SD 256.29]). Within domiciles, foundress numbers ranged from 1 to 22 (median 2 ± SD 2.72). Per capita productivity (total offspring/number of foundresses) ranged from 0.16 to 19.67 (median 4.33 ± SD 3.80), and was associated with the interaction of foundress number (single vs. multiple) and a quadratic effect of domicile volume (GLM with quasipoisson distribution, χ2 = 12.99, df = 1, p = 0.029; Table 1). The quadratic volume term indicated that there was an optimal domicile volume for offspring production, but the significant interaction term meant that this optimum volume differed according to whether domiciles were singly or multiply founded. Specifically, singleton domiciles had a smaller optimum volume than multiply founded domiciles. Over a substantial range of domicile sizes, single females had higher expected per capita reproduction than multiple females (Fig. 1). In the smallest third of domiciles, per capita offspring decreased when multiple foundresses were present, whereas in medium-sized or large domiciles, per capita offspring did not change with foundress number (Fig. 2).
Ovarian status
63 out of 267 dissected foundresses (23.5%) had no developing oocytes. Within domiciles, the proportion of nonreproductive females ranged from 0 to 0.8. Among reproductive females, the volume of developing oocytes ranged from 0.39 × 107 to 3.45 × 107 μm3.
In the minimal model, foundresses’ ovarian status was associated independently with both domicile volume (Fig. 3a) and foundress number (Fig. 3b). Females inhabiting smaller domiciles were generally more likely to be nonreproductive (GLMM with binomial distribution and “domicile id” as a random factor, domicile volume standardized: χ2 = 14.78, df = 1, p < 0.001; Table 2a). However, females in small multiply founded domiciles were more likely to be nonreproductive than females in small singleton domiciles, whereas in large domiciles almost all individuals were reproductive regardless of foundress number; this was evidenced by a significant main effect of foundress number (χ2 = 5.60, df = 1, p = 0.018; Fig. 3a,b). Larger individuals within associations were not more likely than smaller individuals to have developing oocytes (χ2 = 1.49, df = 1, p = 0.22). Analysing these data at the domicile level, using a binomial GLM with “proportion reproductive” as the response variable and “domicile volume” and “foundress number” as predictors (but excluding the individual level variable “pronotum width”), resulted in a qualitatively identical minimal model.
Excluding nonreproductives, oocyte volume among reproductive females was smaller in smaller domiciles (linear mixed model with “domicile id” as a random factor, dropping “domicile volume”, χ2 = 4.84, df = 1, p = 0.027, 4a, Table 2b) and in domiciles containing nonreproductive females (dropping “nonreproductives present in domicile”, χ2 = 6.13, df = 1, p = 0.013, Fig. 4b). Developing oocyte volume did not increase with body size (χ2 = 2.69, df = 1, p = 0.10) nor with the number of foundresses (χ2 = 0.81, df = 1, p = 0.37). Performing this analysis at the domicile level using a linear model with “mean oocyte volume of reproductive females” as the response variable and “domicile volume”, “foundress number” and “presence of nonreproductive females” (but excluding the individual-level variable “pronotum width”) gave a qualitatively identical minimal model.
(a) Mean oocyte volume of reproductive females within a domicile (log transformed), with respect to domicile volume. Double open circles are outliers that were removed for the domicile-level analysis; (b) Oocyte volume of reproductive females (log transformed) with respect to the presence or absence of nonreproductives in the domicile.
Discussion
The relationship between group size and per capita productivity in D. aneurae depended upon the size of the domicile: flat in large domiciles, but negative in small domiciles, such that in small domiciles single females had an advantage over multiple females. In smaller domiciles, and in larger groups, an increasing proportion of females were nonreproductive. Oocyte volume in reproductive females did not decline directly with group size, but instead declined with the presence of nonreproductive females, which in turn tended to occur in smaller domiciles.
Correlational census data must be treated with caution. First, cofounding behaviour may affect the probability of missing data due to whole-domicile failure31,32, something we were unable to determine. Second, the data do not account for the effects of domicile age upon productivity, although we excluded immature and dispersing domiciles as a way of partially accounting for this.
These important caveats notwithstanding, our findings lend support to the idea that resource competition within D. aneurae domiciles at least partly determines both per capita reproduction and reproductive skew. This has previously been suggested for Acacia thrips of the eusocial, gall-inducing species22, which form a sister clade to phyllode-gluers such as D. aneurae21. In those species, reproductive skew has increased as galls have become progressively smaller over evolutionary time22,24, suggesting that competition for resources within galls has facilitated the evolution of high reproductive skew (and ultimately of reproductively subordinate soldier castes).
By implicitly similar reasoning, Crespi et al.21 suggested that domicile architecture may provide a context for social evolution in Dunatothrips. Their basis for this suggestion was that the more-or-less nonsocial species such as D. gloius and D. armatus tend to construct simple, fairly uniform domiciles out of two crossed phyllodes, while the cofounding species, D. aneurae and D. vestitor, use many more phyllodes to construct looser, more irregular domiciles with much greater size variance; additionally, domicile extensibility may also be important33,34.
In D. aneurae, we have shown that similar effects appear to be evident across the range of domicile sizes within a single population. In small domiciles, females did better on their own than in groups — to the extent that some group-living females were actually nonreproductive. It is unlikely that these nonreproductive females were simply callow or teneral immature offspring of one of the other foundresses, because (1) they were dealate (as were all females included in our analyses), and females retain wings until after dispersal and domicile construction27; (2) they occurred in small domiciles of all ages — commonly occurring in domiciles that contained only dealate females and eggs but no nymphs; and (3) they were never seen in large domiciles (Fig. 4), and there is no a priori reason to expect juveniles to be absent from large domiciles.
By contrast, in large domiciles, individuals did equally well regardless of female numbers, suggesting that competition for resources was not a limiting factor upon reproduction. A similar effect was shown in striped mice12, which generally live in groups, except in the breeding season, when intense reproductive competition means they form groups only at high population density. Generally, fecundity of plant-exploiting insects can vary with the size or quality of the resource35. In social groups, reproduction is often closely linked to resources10,36 and within-group competition is frequently an agent of reproductive suppression in both vertebrate and invertebrate societiesreviewed in17,37,38. In species that cohabit galls, resource competition can limit reproduction for inhabitants39. More generally, nest morphology has been implicated in social evolution in a variety of taxa22,40,41,42,43.
Hence it may be that breeding in D. aneurae is despotic or communal, depending on the extent of competition for resources. The question remains (as for the gall-inducing thrips): by what mechanism does such resource competition operate? For example, the negative association between resource size and skew that we observe here would be expected under several current predictive frameworks. First, this association is consistent with the intuitively plausible idea that resources related to the size of the domicile limit reproduction for inhabitants, such that some end up nonreproductive. In D. aneurae, a good candidate for such a limiting resource is the feeding substrate, i.e. the phyllode surface within the domicile. Individuals are thought to feed entirely within their domiciles, perhaps due to highly desiccating conditions outside30 and have never been documented or observed feeding outside; the phyllode surface within mature domiciles is yellow and necrotic compared to fresh green tissue immediately outside (JDJG, pers. obs.). Thus it seems reasonable to suppose that in small domiciles this surface area may limit reproduction. Alternatively, space within the domicile may be a limiting resource.
Secondly, however, our data are also consistent with the idea that reproductive skew is the result of a “tug of war” between dominants and subordinates, whereby larger resources (whether limiting to reproduction or not) are more difficult to monopolise by any given individual17,44. Thirdly, reproductive skew may reflect the extent of “staying incentives” offered by dominants to entice subordinates not to leave the group, predicting that larger incentives are required where each subordinate contributes proportionally less to group productivity (e.g. in larger groups, supported by better resources, where subordinate effort is diluted). Finally, skew may reflect “peace incentives” to induce subordinates not to escalate conflict19,20, which predicts lower skew on better resources where subordinates are stronger and thus more likely to mount a challenge to the dominant.
Thus, a small domicile may (1) provide insufficient resources for all potentially reproductive group members to breed, resulting in intra-domicile competition in which the losers become nonreproductive; (2) provide a small enough arena in which one or a few individuals may be effective in monopolising resources, suppressing the reproduction of others; (3) support a small enough group that a subordinate is capable of making a difference to her kin-group’s fitness which on its own outweighs the benefits of breeding independently, so she does not require a reproductive incentive to stay; or (4) support a small enough group that the threat of escalated conflict does not require a peace incentive and the dominant is able to suppress the reproduction of other members. Teasing apart these alternatives will require detailed experiments.
In large domiciles, despite abundant resources single females tended to fare less well than females in groups. It may be that a single female cannot fully utilize the space available in a large domicile (for example, the median total offspring observed across all singleton domiciles was 6 [±IQR 2–10; range 0–26], compared to the offspring potentially attainable in the largest 10% of all domiciles, which was a median of 43 total offspring [±IQR 10–58; range 0–87]). In large domiciles, single females may incur deleterious costs of construction and maintenance45, reducing their reproductive output. Under these circumstances, potential extra females may be beneficial as they may share maintenance and/or defence costs without imposing costs of competition46.
What is the role of nonreproductive dealate females in D. aneurae? With correlational snapshot data, conclusions are inevitably tentative and separating the various possibilities will require further experimental data. Nonreproductive dealate females may be mature, dispersed females that are not yet ready to lay. Eggs of D. aneurae are exceptionally large for Acacia thrips (max size of mature oocyte in this study = 2.7 × 107 μm3, which is approx. 3× the largest median egg size for gall-inducing species examined by Kranz47; see her Fig. 2) and females may therefore need to build up resources for a long time before being able to lay. We note that this would make competition for such resources all the more important in determining who breeds at any given moment. Alternatively, nonreproductive females may be ready to lay, but waiting for an opportunity to inherit a breeding position within the domicile48,49.
One possibility is that they act as nonreproductive helpers to related nestmates, in which case D. aneurae could be thought of as a cooperative breeder. However, developing oocyte volume of breeders went down, not up, when nonreproductive females were present in the domicile, even when controlling for domicile size (Fig. 4a). Whilst we reiterate that this correlative result requires experimental data for confirmation, the data suggest that nonreproductive individuals may not have a positive effect upon breeders’ fecundity, or at least on their ovarian status. They may yet have unmeasured positive effects upon survival of offspring via “assured fitness returns”50,51, or by “lightening breeders’ load”52 — for example by helping repair domicile damage to maintain humidity as suggested in ref.30 or in a hygienic role such as maintaining middens27.
Given the apparent negative effect of nonbreeding females upon breeders’ fecundity, why would breeding females tolerate nonbreeding nestmates? D. aneurae tolerates both foreign conspecifics27 and inquilines of other species29; in this context, perhaps tolerance of nonreproductive nestmates is unsurprising. Nevertheless females are at least capable of evicting males after mating27, although this behaviour may carry costs. Any conflict of interest over acceptance may be asymmetrical; an individual may gain more by staying (or joining) than the residents lose by accepting her1,53,54. Alternatively, as above, nonreproductive females may be tolerated because they confer unmeasured benefits to breeders such as helping with domicile repair or performing hygienic functions.
Why would multiple foundresses make a small domicile, when in small domiciles females may be forced to become nonreproductive or to wait to breed — and, so doing, could even reduce productivity of breeders? One possible scenario would be if small domiciles were typically founded singly and then joined by others later, parasitically, after construction54,55. Preliminary lab and field data show that, while females typically cooperate from the moment of domicile initiation25, a substantial proportion of established domiciles are also joined by additional females (Gilbert & Simpson, MS in prep). Whether joiners tend to be nonreproductive remains to be established, but one intuitively appealing hypothesis for future research is that vagrant (and presumably unrelated) females without domiciles may sometimes join established domiciles parasitically and compete with foundresses, only becoming reproductive if there are enough resources (i.e. if the domicile is large enough).
Another (nonexclusive) possibility is that cofounding females may not know exactly the eventual size of the domicile before they begin construction. Nothing is yet known about interactions at the moment of domicile formation. It is likely that females have partial but not full control over the eventual size of the domicile they build, because of mechanical constraints imposed by the specific phyllodes they choose to tie together, which are typically much larger than the thrips themselves. Thus, a female or group of females may end up in a domicile larger or smaller than optimal56. Single females may have less control over domicile location, size and shape than groups of cooperating females — increasing numbers of foundresses are associated with reduced variance in the dimensions of phyllodes used to build domiciles (Gilbert and Simpson, MS in prep).
Future directions
Experimental data would clearly be necessary to test conclusively how within-group fitness is causally affected by competition10 and should now be a priority for research. In addition, data on genetic relatedness and offspring maternity will be essential to evaluate the costs and benefits of social behaviour in D. aneurae in the context of kin selection. Within-group relatedness can demonstrably affect reproductive skew57,58 and may be a critical factor determining whether or not thrips benefit from competing with nestmates over reproduction. One analysis demonstrated that D. aneurae has a mixture of high and low relatedness within domiciles28 consistent with their having later been observed both inbreeding and outbreeding27 and both cofounding and joining (Gilbert & Simpson, MS in prep); preliminary analyses on our study population reveal that relatedness is mostly very high (LA Rollins, JDJ Gilbert, SJ Simpson, unpublished data). Finally, to understand the dynamics of competition and cooperation before, during and after domicile construction, it will be important to determine how single and multiple foundresses choose and exploit nesting sites, how much control they have over domicile size, and the significance and dynamics of domicile-joining behaviour.
Methods
Dunatothrips aneurae domiciles occur predominantly on terminal phyllodes of narrow-phyllode varieties of A. aneura27. Domiciles’ principal function is to reduce desiccation in the arid environment30. Construction is aseasonal, and appears to require male presence27, although founding males are seldom found in field domiciles26 After construction, D. aneurae foundresses cast off their wings and produce one generation of offspring25. Most offspring disperse but indirect evidence suggests some may reproduce in the natal domicile28. Domiciles may be extended over time59 although this was rare in our population; most are simple and consist of a single chamber (Gilbert & Simpson, unpublished data).
In fieldwork trips between September 2011 and October 2013 we collected 513 D. aneurae domiciles from thin-phyllode variants of A. aneura on the Fowlers Gap property, approx. 110 km N of Broken Hill, Australia (see ref.27 for location details) and placed them in a fridge at 4 °C until dissection under a binocular microscope. Most domiciles were dissected within 3 days of collection; 91 domiciles were placed in a freezer at −20 °C and dissected approx. 1 year later (excluding these domiciles from analysis had no appreciable effect upon results).
Domicile volume, foundress number and per capita productivity
We estimated domicile volume as a simple cuboid, i.e. length × width × depth at their largest dimensions, ignoring any convolutions. Most domiciles were of a reasonably simple shape that warranted this assumption.
We counted domicile inhabitants and classified them as reproductive foundresses (dealate females26,27), offspring of all stages up to alate adults, or, rarely, adult males. Adult males do not lose their wings upon reproduction and so may potentially be confused with adult offspring. However, adult males are very rare in active D. aneurae domiciles25,26,27. Accordingly, any single adult males that occurred alongside alate female adults, plus multiple adult males occurring in the same domicile, were assumed to be male alate offspring. Single adult males found in domiciles with only dealate females and eggs or very young larvae present were assumed to be reproductive males and excluded from analysis. We also counted eggs and classified them as hatched or unhatched. We excluded from analysis any domiciles that were under construction, which contained no offspring and/or no foundress, and domiciles with only adult offspring (from which some offspring may have already dispersed).
We calculated total domicile offspring as the sum of the number of live offspring in each domicile and the number of unhatched eggs present. We did not attempt to count failed offspring within domiciles, nor the probability of whole-domicile failure. Per capita offspring was calculated simply as the total offspring divided by the number of foundresses. We analysed per capita offspring as a function of domicile volume and foundress number using a generalized linear model (GLM) with a quasipoisson distribution to account for overdispersion.
Ovarian status
In a subset of domiciles (ndomiciles = 164, nfoundresses = 267), foundresses were killed by immersing in 100% ethanol for 1 minute, and were dissected immediately in water. We measured the pronotum width using an eyepiece reticle. The extent of ovary development could be clearly classified as developed or undeveloped (Fig. 5). In developed ovaries, we measured the length and width of any developing oocytes. The volume of each oocyte was calculated as an ellipsoid (π × length × (width/2)2) and the resulting volumes were summed to give the total volume of developing oocytes in each ovary.
We analysed ovarian status (developed or undeveloped) as a function of domicile volume, foundress number and body size (pronotum width) using a generalized linear mixed model (GLMM) with a binomial distribution and “domicile ID” as a random factor. The probability of a given female being nonreproductive (i.e. with no developing oocytes) can be viewed as an estimate of skew — although note that estimates based on ovarian dissections represent a conservative minimum estimate. Genetic data would be necessary to test whether those females that do have developing oocytes are actually ovipositing within the domicile24,60,61.
Among the subset of females that were reproductive, we analysed the total volume of developing oocytes using a linear mixed model with domicile volume, foundress number, pronotum width and the presence/absence of nonreproductive females as predictor variables and “domicile ID” as a random factor. All analyses were carried out in R 3.2.162.
Data accessibility
All relevant datasets are provided as supplementary material. Additionally all data are archived in Dryad under https://doi.org/10.5061/dryad.273k8.
References
Krause, J. & Ruxton, G. D. Living in groups (Oxford University Press, 2002).
Emlen, S. T. The Evolution of Helping. I. An Ecological Constraints Model. Am. Nat. 119, 29–39 (1982).
Herbers, J. M. Nest Site Limitation and Facultative Polygyny in the Ant Leptothorax longispinosus. Behav. Ecol. Sociobiol. 19, 115–122 (1986).
McCorquodale, D. B. Soil softness, nest initiation and nest sharing in the wasp, Cerceris antipodes (Hymenoptera: Sphecidae). Ecol. Ent. 14, 191–196 (1989).
Komdeur, J. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358, 493–495 (1992).
Field, J., Foster, W., Shreeves, G. & Sumner, S. Ecological constraints on independent nesting in facultatively eusocial hover wasps. Proc. Biol. Sci 265, 973–977 (1998).
Koenig, W. D., Pitelka, F. A., Carmen, W. J., Mumme, R. L. & Stanback, M. T. The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 67, 111–150 (1992).
Wong, M. Y. L. Ecological constraints and benefits of philopatry promote group-living in a social but non-cooperatively breeding fish. Proc. Biol. Sci. 277, 353–358 (2010).
Lee, K. P. et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 105, 2498–2503 (2008).
Salomon, M., Mayntz, D. & Lubin, Y. Colony nutrition skews reproduction in a social spider. Behav. Ecol. 19, 605–611 (2008).
Korb, J. & Schmidinger, S. Help or Disperse? Cooperation in Termites Influenced by Food Conditions. Behav. Ecol. Sociobiol. 56, 89–95 (2004).
Schoepf, I. & Schradin, C. Better off alone! Reproductive competition and ecological constraints determine sociality in the African striped mouse (Rhabdomys pumilio). J. Anim. Ecol. 81, 649–656 (2012).
Keller, L. & Reeve, H. K. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 9, 98–102 (1994).
Choe, J. C. & Crespi, B. J. The evolution of social behaviour in insects and arachnids (Cambridge University Press, 1997).
Solomon, N. G. & French, J. A. Cooperative breeding in mammals (Cambridge University Press, 1997).
Koenig, W. D. & Dickinson, J. L. Ecology and evolution of cooperative breeding in birds (Cambridge University Press, 2004).
Clutton-Brock, T. H. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 13, 288–292 (1998).
Reeve, H. K. & Emlen, S. T. Reproductive skew and group size: an N-person staying incentive model. Behav. Ecol. 11, 640–647 (2000).
Reeve, H. K. & Ratnieks, F. Queen-queen conflicts in polygynous societies: mutual tolerance and reproductive skew. Queen number and sociality in insects. Oxford University Press, Oxford 45, 85 (1993).
Cant, M. A., English, S., Reeve, H. K. & Field, J. Escalated conflict in a social hierarchy. Proc. Biol. Sci. 273, 2977–2984 (2006).
Crespi, B. J., Morris, D. C., Mound, L. A. Evolution of ecological and behavioural diversity: Australian Acacia thrips as model organisms (Australian Biological Resources Study, 2004).
Wills, T. E., Chapman, T. W., Kranz, B. D. & Schwarz, M. P. Reproductive division of labour coevolves with gall size in Australian thrips with soldiers. Naturwissenschaften 88, 526–529 (2001).
Kranz, B. D. et al. A fully reproductive fighting morph in a soldier clade of gall-inducing thrips (Oncothrips morrisi). Behav. Ecol. Sociobiol. 50, 151–161 (2001).
Chapman, T. W. et al. The evolution of soldier reproduction in social thrips. Behav. Ecol. 13, 519–525 (2002).
Morris, D. C., Schwarz, M. P. & Crespi, B. J. Pleometrosis in phyllode-glueing thrips (Thysanoptera: Phlaeothripidae) on Australian Acacia. Biol. J. Linn. Soc. Lond. 75, 467–474 (2002).
Bono, J. M. & Crespi, B. J. Costs and benefits of joint colony founding in Australian Acacia thrips. Insectes Soc. 53, 489–495 (2006).
Gilbert, J. D. J. & Simpson, S. J. Natural history and behaviour of Dunatothrips aneurae Mound (Thysanoptera: Phlaeothripidae), a phyllode-gluing thrips with facultative pleometrosis. Biol J. Linn. Soc. Lond. 109, 802–816 (2013).
Bono, J. M. & Crespi, B. J. Cofoundress relatedness and group productivity in colonies of social Dunatothrips (Insecta: Thysanoptera) on Australian Acacia. Behav. Ecol. Sociobiol. 62, 1489–1498 (2008).
Gilbert, J. D. J., Mound, L. A. & Simpson, S. J. Biology of a new species of socially parasitic thrips (Thysanoptera: Phlaeothripidae) inside Dunatothrips nests, with evolutionary implications for inquilinism in thrips. Biol. J. Linn. Soc. Lond. 107, 112–122 (2012).
Gilbert, J. D. J. Thrips domiciles protect larvae from desiccation in an arid environment. Behav. Ecol. 25, 1338–1346 (2014).
Michener, C. D. Reproductive efficiency in relation to colony size in hymenopterous societies. Insectes Soc. 11, 317–341 (1964).
Strassmann, J. E. & Queller, D. C. In: The Genetics of Social Evolution (eds. Breed, M. & Page, R.) 81–101 (Westview Press, 1989).
Buskirk, R. E. in Social insects, vol. 2 (ed. Hermann, H.R.) 281–367 (Academic Press, 1981).
Ward, P. I. & Enders, M. M. Conflict and cooperation in the group feeding of the social spider Stegodyphus mimosarum. Behaviour 94, 167–182 (1985).
Whitham, T. G. Habitat selection by Pemphigus aphids in response to response limitation and competition. Ecology 59, 1164–1176 (1978).
Clutton-Brock, T. H. et al. Reproduction and survival of suricates (Suricata suricatta) in the southern Kalahari. Afr. J. Ecol. 37, 69–80 (1999).
Peeters, C. & Higashi, S. Reproductive dominance controlled by mutilation in the queenless ant Diacamma australe. Naturwissenschaften 76, 177–180 (1989).
Young, A. J. et al. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl. Acad. Sci. USA 103, 12005–12010 (2006).
Weis, A. E., Walton, R. & Crego, C. L. Reactive plant tissue sites and the population biology of gall makers. Annu. Rev. Entomol. 33, 467–486 (1988).
Ozaki, K. Effects of gall volume on survival and fecundity of gall-making aphids Adelges japonicus (Homoptera: Adelgidae). Res. Popul. Ecol. 35, 273–284 (1993).
Hansell, M. H. in Natural history and evolution of paper-wasps (eds. Turillazzi, S. & West-Eberhard, M. J.) 272–289 (Oxford University Press, 1996).
Shellman-Reeve, J. S. in The evolution of social behavior in insects and arachnids (eds. Crespi B. J. & Choe J. C.) 52–93 (Cambridge University Press, 1997).
Hogendoorn, K., Watiniasih, N. L. & Schwarz, M. P. Extended alloparental care in the almost solitary bee Exoneurella eremophila (Hymenoptera: Apidae). Behav. Ecol. Sociobiol. 50, 275–282 (2001).
Reeve, H. K., Emlen, S. T. & Keller, L. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 9, 267–278 (1998).
Kvarnemo, C. Size-assortative nest choice in the absence of competition in males of the sand goby. Pomatoschistus minutus. Environ. Biol. Fishes 43, 233–239 (1995).
Scott, M. P. Reproductive dominance and differential ovicide in the communally breeding burying beetle Nicrophorus tomentosus. Behav. Ecol. Sociobiol. 40, 313–320 (1997).
Kranz, B. D. Egg size and reproductive allocation in eusocial thrips. Behav. Ecol. 16, 779–787 (2005).
Kokko, H. & Johnstone, R. A. Social queuing in animal societies: a dynamic model of reproductive skew. Proc. Biol. Sci. 266, 571–578 (1999).
Leadbeater, E., Carruthers, J. M., Green, J. P., Rosser, N. S. & Field, J. Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876 (2011).
Gadagkar, R. Evolution of eusociality: the advantage of assured fitness returns. Phil. Trans. Biol. Sci. 329, 17–25 (1990).
Lucas, E. R. & Field, J. Assured fitness returns in a social wasp with no worker caste. Proc. Biol. Sci. 278, 2991–2995 (2011).
Russell, A. F., Langmore, N. E., Cockburn, A., Astheimer, L. B. & Kilner, R. M. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944 (2007).
Sibly, R. M. Optimal group size is unstable. Anim. Behav. https://doi.org/10.1016/S0003-3472(83)80250-80254 (1983).
Higashi, M. & Yamamura, N. What determines animal group size? Insider-outsider conflict and its resolution. Am. Nat. 142, 553–563 (1993).
Ens, B. J., Weissing, F. J. & Drent, R. H. The despotic distribution and deferred maturity: two sides of the same coin. Am. Nat. 146, 625–650 (1995).
Franks, N. R., Dornhaus, A., Best, C. S. & Jones, E. L. Decision making by small and large house-hunting ant colonies: one size fits all. Anim. Behav. 72, 611–616 (2006).
Lucas, E. R., Martins, R. P. & Field, J. Reproductive skew is highly variable and correlated with genetic relatedness in a social apoid wasp. Behav. Ecol (2011).
Harradine, S. L., Gardner, M. G. & Schwarz, M. P. Kinship in a social bee mediates ovarian differentiation and has implications for reproductive skew theories. Anim. Behav. 84, 611–618 (2012).
Mound, L. A. & Morris, D. C. Domicile constructing phlaeothripine Thysanoptera from Acacia phyllodes in Australia: Dunatothrips Moulton and Sartrithrips gen.n., with a key to associated genera. Syst. Entomol. 26, 401–419 (2001).
Cini, A., Meconcelli, S. & Cervo, R. Ovarian indexes as indicators of reproductive investment and egg-laying activity in social insects: a comparison among methods. Insectes Soc. 60, 393–402 (2013).
Green, J. P., Cant, M. A. & Field, J. Using social parasitism to test reproductive skew models in a primitively eusocial wasp. Proc. Biol. Sci. 281, 20141206 (2014).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria., http://www.R-project.org/ (2015).
Acknowledgements
We thank Laurence Mound for invaluable assistance with thrips identification, logistics, help in the field and a wealth of general advice, K. Leggett and G. and V. Dowling for assistance with logistics, and J. Field and L. E. Browning for helpful discussions and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
J.D.J.G. conceived the study, designed the work, carried out field and lab data collection, performed the statistical analyses and wrote the manuscript. A.W. collected key field and lab data. S.J.S. helped to conceive the study and to draft the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gilbert, J.D.J., Wells, A. & Simpson, S.J. Skew in ovarian activation depends on domicile size in phyllode-glueing thrips. Sci Rep 8, 3597 (2018). https://doi.org/10.1038/s41598-018-21635-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21635-z
- Springer Nature Limited