Abstract
Influenza virus infection remains a major global health problem and requires a universal vaccine with broad protection against different subtypes as well as a rapid-response vaccine to provide immediate protection in the event of an epidemic outbreak. Here, we show that intranasal administration of probiotic Escherichia coli Nissle 1917 activates innate immunity in the respiratory tract and provides immediate protection against influenza virus infection within 1 day. Based on this vehicle, a recombinant strain is engineered to express and secret five tandem repeats of the extracellular domain of matrix protein 2 from different influenza virus subtypes. Intranasal vaccination with this strain induces durable humoral and mucosal responses in the respiratory tract, and provides broad protection against the lethal challenge of divergent influenza viruses in female BALB/c mice. Our findings highlight a promising delivery platform for developing mucosal vaccines that provide immediate and sustained protection against respiratory pathogens.
Similar content being viewed by others
Introduction
Influenza virus infection remains a major global health problem, resulting in significant morbidity and mortality each year1. Vaccination is one of the most effective public health interventions to combat influenza2. However, current seasonal inactivated influenza vaccine only protects against a narrow range of virus strains3. There is an urgent need for “universal” influenza vaccines that provide comprehensive protection against influenza viruses4,5. In addition, current vaccines require multiple doses to achieve full efficacy and take time to activate the host immunity3, so a vaccine that provides immediate protection at the onset of an epidemic is needed.
Efforts to develop universal influenza vaccines have included experimental vaccines that specifically target the extracellular domain of matrix protein 2 (M2e)6,7, neuraminidase (NA)8,9, or antigenically conserved epitopes on the hemagglutinin (HA) head and stalk10,11. Among these targets, the M2e antigen is remarkably conserved in influenza A virus6. The M2e-specific antibodies protect against infection through both Fc receptor (FcR)-mediated antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC)12. Therefore, M2e has been employed as an ideal target for the development of universal influenza A vaccines by fusing with flagellum protein13, hepatitis B virus core (HBc)14, a specific dendritic cell subset7 or nanoparticle cores15.
To combat emerging threats from respiratory pathogens, vaccines that can elicit both mucosal and innate immunity are urgently needed16,17. Compared to intramuscular vaccines, nasally administered vaccines have the advantage of inducing local mucosal immune responses that can block infection and transmission of respiratory pathogens18,19. In the last decade, there has been increasing interest in the use of live bacterial vehicles for antigen delivery to local mucosal tissues20 because the lipopolysaccharides in Gram-negative bacteria or lipoteichoic acid in Gram-positive bacteria could be recognized by pattern recognition receptors (PRRs), leading to non-specific activation of the innate immune response in the respiratory tract to form an immediate and broad-spectrum protective barrier against pathogens21,22. However, bacterial surface modules would elicit different immune responses22, suggesting that the bacterium used as a mucosal delivery vehicle should be carefully tested. Regardless, these innate immune responses and their capacity to deliver antigens to activate the adaptive immune system make living microorganisms as potent vehicles for stimulating both immediate and long-term protection against respiratory pathogens.
Escherichia coli Nissle 1917 (EcN), a genetically tractable probiotic with well-established safety in humans, is emerging as a favored chassis for the treatment of immune-related and metabolic diseases in mouse models and clinical trials23,24,25,26,27. Importantly, EcNs immunomodulatory properties, particularly its ability to modulate chemokine expression via its K5 capsule, suggest its potential as an effective agent for enhancing host immune defenses28,29. Nonetheless, the application of EcN as a mucosal vaccine delivery vehicle warrants empirical validation.
In this study, we demonstrated that intranasal administration of EcN effectively activated lung innate immune responses and conferred immediate protection against influenza virus infection in female BALB/c mice. Based on these findings, we engineered EcN to secrete five tandem repeats of the M2e antigen from different influenza virus subtypes and found that intranasal vaccination with EcN-5M2e provided effective cross protection against the lethal challenge of divergent influenza A viruses. These findings endorse EcN as a promising platform for the development of mucosal vaccines that offer both immediate and enduring protection against respiratory pathogens.
Results
Intranasal administration of EcN activates innate immunity to protect against influenza virus infection
To evaluate the potential of using the probiotic EcN as a vehicle for the development of a vaccine against the respiratory pathogens, we first examined the distribution of EcN in mice by intranasal administration. While the majority of bacteria were observed in the gastrointestinal tract, relative high level of EcN was also observed in lung tissue for a short time (<12 h) (Fig. 1a, b), and decreasing amount of EcN was detected in lung tissue within 2 weeks (Fig. 1c). We then examined the potential of EcN to cause infection by intranasal administration and monitored the mice for 14 days after EcN exposure (Fig. 1d). Although a slight decrease in body weight was observed in the EcN group 1–2 days after administration, no systematic infection was evident based on routine blood tests (Fig. 1e, f). Some inflammatory cell infiltration was observed in the lung 1 and 3 days after treatment with PBS or EcN, but this inflammation was disappeared by day 7 (Supplementary Fig. 1a). Importantly, these responses were restricted to the lung, as no EcN was detected in the heart, liver, spleen or kidney (Fig. 1a; Supplementary Fig. 1b).
a Representative in vivo IVIS images showing mCherry-labeled EcN (EcN-mCherry, 1 × 108 CFU/10 μL) distribution in key organs post-intranasal delivery at specific times. Organs: Lu (lung and trachea section), St (stomach), Si (small intestine), Li (large intestine), H (heart), L (liver), S (spleen), K (kidney). b Quantitative analysis of relative fluorescence intensity of mCherry-labeled EcN in lungs at the indicated time points (n = 3/group). c Colony forming units (CFU) of bacteria in the lung tissue of mice at the indicated time points after intranasal administration of EcN-mCherry (n = 4/group). d Schematic representation of the experimental protocol. PBS represents the control group and EcN represents the group treated with wild-type EcN strain. e The body weight of mice was monitored for 7 days after intranasal administration with EcN (n = 5/group). This experiment was repeated twice independently. f Hemogram indices of the treated mice at the indicated time points (Day1: PBS n = 8, EcN n = 8; Day3: PBS n = 10, EcN n = 10; Day7: PBS n = 7, EcN n = 8; Day14: PBS n = 6, EcN n = 6). g Lung cytokine levels in PBS vs. EcN-treated mice over 1, 3, 7, and 14 days (Day1: PBS n = 3, EcN n = 5; Day3, Day7 and Day14: PBS n = 4, EcN n = 4). Data are presented as mean ± SEM. Statistical significance was analyzed by two-tailed unpaired t-test. Source data are provided as a Source Data file.
Considering that colonization of microorganisms in the lung can activate the local immune system30, we analyzed cytokine changes in the lung 1, 3, 7 and 14 days after EcN exposure. EcN-treated mice showed increased expression of anti-infection interferons (IFN-α, IFN-β and IFN-γ) in lung tissue after 1, 3, 7 and 14 days compared to PBS-treated mice (Fig. 1g). Furthermore, pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-12, and IL-18) and various chemokines (e.g. CCL2 and CCL5) were all upregulated in the lungs of EcN-treated mice (Fig. 1g; Supplementary Fig. 2).
After confirming the EcN-induced activation of key antiviral innate responses in lung tissue, we examined the protective effect against influenza virus infection (Fig. 2a). Mice were pre-administered with EcN and the next day infected with 3 × LD50 of PR8 (H1N1) influenza virus (Fig. 2a). Although the body weight of the EcN-treated group initially decreased slightly, it gradually recovered over the following days (Fig. 2b). Notably, all mice in the EcN group survived, whereas all mice in the PBS group died (Fig. 2c). We next investigated whether EcN can protect against influenza virus infection after longer periods of administration. At 3, 7 and 14 days after pretreatment with EcN, we monitored morbidity and mortality in mice treated with PR8 influenza virus (Fig. 2a). Protection rates remained at 100%, 75% and 30% in the 3 day, 7 day and 14 day EcN groups, respectively (Fig. 2b, c), which correlated with the levels of antiviral cytokines observed in lung tissue (Fig. 1g). Taken together, these data highlight the ability of intranasal administration of EcN to activate innate immunity in the respiratory tract and provide immediate protection against influenza virus infection.
a Schematic representation of the experimental procedure for infection with A/Puerto Rico/8/1934 (H1N1) after intranasal treatment with EcN or PBS (D1 group: PBS n = 8, EcN n = 8; D3 group: PBS n = 6, EcN n = 6; D7 group: PBS n = 6, EcN n = 8; D14 group: PBS n = 6, EcN n = 10). Weight loss (b) and survival (c) were monitored for 14 days. Data are presented as mean ± SEM. Source data are provided as a Source Data file.
Rational design of EcN-based universal influenza vaccine EcN-5M2e
Having confirmed that EcN activates innate immunity to protect against influenza virus infection, we next aim to develop a universal influenza vaccine based on EcN. We focused on expressing the conserved M2e antigen in EcN to stimulate adaptive immunity. To identify the representative M2e fragments, we performed a phylogenetic analysis of M2e from homo-derived influenza A viruses. Fragments were selected that represent different branches of evolution and correspond to subtypes that have caused pandemic infections or pose a potential risk of transmission31 (Fig. 3a). To increase the immunogenicity of the M2e fragment as previously reported6,32,33, the M2e sequences from H1N1, H2N2, H3N2, H5N1 and H7N9 were concatenated to form the 5M2e antigen (Fig. 3b). The natural cysteines in M2e (C17 and C19) were replaced with serine to prevent the formation of disulfide bonds that can lead to protein aggregation. This 5M2e protein was well expressed in E. coli BL21(DE3) and successfully recognized by the M2e-specific 14C2 antibody (Supplementary Fig. 3). Immunization with the 5M2e protein generated IgG antibodies that specifically recognized the M2e peptide of H1N1, H2N2, H3N2, H5N1 or H7N9, which were incorporated into the design of 5M2e (Fig. 3c). Importantly, the antibodies induced by the 5M2e antigen showed broad-spectrum recognition ability as they recognized M2e peptides outside the design (H6N6, H9N2, H13N2, H17N10 and H18N11) (Fig. 3c).
a Phylogenetic tree for M2e peptides of human-infecting influenza A viruses created using iqtree. b Cartoon model of the 5M2e design and the sequences of M2e from different influenza A virus strains. c Immunogenicity analysis of 5M2e protein. Purified 5M2e protein (10 μg, plus aluminum adjuvant) was immunized subcutaneously three times at 2 weeks intervals (primer-boost-boost). The endpoint titer of IgG to different M2e peptides in serum 1 week after each immunization was determined by ELISA (n = 3/group). Data are presented as mean ± SEM. Statistical significance was analyzed by two-tailed unpaired t-test. d Schematic illustration of the structure of the manipulated probiotic EcN-5M2e vaccine. e Western blot analysis of extracellular 5M2e protein by anti-His or M2e-specific monoclonal 14C2 antibodies. The experiment was repeated three times independently with similar results. Source data are provided as a Source Data file.
An important concern in the development of probiotics is to avoid the use of antibiotic resistance genes in order to meet the requirements for their application in the medical and food industries34. To this end, we used a complementary diaminopimelic acid (dap) plasmid system to express 5M2e without an antibiotic resistance gene in EcN. We first constructed an EcN strain (EcNΔdapB) with a deletion of the dapB gene, which prevents the biosynthesis of the important cell wall component diaminopimelic acid and potentially impairs survival in a mammalian host environment since dap is not produced by eukaryotes35. We then generated a plasmid named pKT-5M2e-HlyABD (Supplementary Fig. 4a) to express DapB and secret 5M2e using the hemolysin A secretion system. This plasmid was then transformed into EcNΔdapB, resulting in the development of the vaccine strain EcN-5M2e (Fig. 3d). In this system, a complementary dapB gene was inserted into pKT-5M2e-HlyABD to enable growth of the EcNΔdapB strain (Supplementary Fig. 4b). Secreted 5M2e protein in the culture supernatant was detected for the EcN-5M2e strain using both His-tag and 14C2 monoclonal antibodies (Fig. 3e). Furthermore, the EcN-5M2e strain showed stable proliferation and passage (Supplementary Fig. 4c), suggesting its suitability for vaccine evaluation.
Intranasal immunization with EcN-5M2e elicits effective humoral, mucosal and T cell responses
To evaluate the immunogenicity of EcN-5M2e, mice were immunized three times intranasally with 108 CFU of EcN-5M2e, EcN-vec, or PBS, and serum samples were collected 7 days after each immunization for further analysis (Fig. 4a). After the 1st and 2nd boosts, the EcN-5M2e triggered a significant increase in M2e-specific IgG antibodies as tested by H1N1-specific M2e peptide (Fig. 4b). Importantly, EcN-5M2e immune sera can bind to the M2 protein expressed by influenza virus-infected cells (Supplementary Fig. 5), suggesting the possible contribution of these sera in virus clearance by promoting ADCC12. Compared to the other groups, the mice immunized intranasally with EcN-5M2e showed significantly higher titers of M2e-specific IgG1 and IgG2a after the 2nd booster vaccination, indicating the induction of both Th1-type and Th2-type immune responses in vivo (Fig. 4c, d).
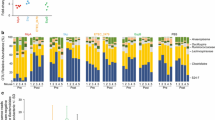
a Schematic illustration of immunization and sampling in the mouse model. Mice were immunized intranasally with EcN-vec, EcN-5M2e, or PBS control. b Endpoint titers of IgG against H1N1-M2e peptide in serum samples collected one week after primer, the 1st or 2nd booster vaccination as determined by ELISA (n = 10/group). c, d Endpoint titers of IgG1 (c) and IgG2a (d) against H1N1-M2e peptide. Sera were collected one week after the 2nd booster vaccination (n = 10/group). e–g Mucosal IgA levels of nasal lavage fluid (NALF) (e) (n = 5/group), bronchoalveolar lavage fluid (BALF) (f) (n = 5/group) and serum (g) (n = 8/group) samples collected 2 weeks after the 2nd booster vaccination. Absorbance values at 450 nm (Abs450) in the ELISA assay against H1N1-M2e peptide were recorded. Serum samples were diluted 10-fold in this test. h Percentages of M2e-specific CD4+ T cells producing IFN-γ or IL-2 (n = 5/group). i Percentages of M2e-specific CD8+ T cells producing IFN-γ or IL-2 (n = 5/group). Panels (h, i) are representative data of 2 independent tests. j Cross-binding ability of IgG, IgG1, IgG2a against M2e of various influenza viruses in serum samples taken one week after the 2nd booster vaccination (n = 10/group). The endpoint titer of each sample is displayed. k Cross-binding ability of IgA against M2e of various influenza viruses in NALF (n = 5/group), BALF (n = 5/group) and 10-fold diluted serum (n = 8/group). Data are presented as mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparison test (panels b–i) or two-tailed unpaired t-test (panels j, k). Source data are provided as a Source Data file.
Given that the mucosal surfaces of the respiratory tract serve as major entry points for influenza viruses36, the production of both mucosal IgG and IgA is conducive to effective protection. We next focused on the immune responses that EcN-5M2e elicits in mucosa tissues. Lavage fluids were collected 2 weeks after the 2nd boost. Intranasal immunization with EcN-5M2e resulted in significant H1N1-M2e-specific mucosal IgA responses in nasal lavage fluid (NALF), bronchoalveolar lavage fluid (BALF), and serum samples (Fig. 4e–g). In addition, mucosal IgA was also detected in the intestinal lavage fluid (ILF) (Supplementary Fig. 6a), which is in consistent with the distribution of EcN-5M2e in the intestine. Additionally, IgG was also detected in NALF, BALF and ILF (Supplementary Fig. 6b–d).
We also examined the M2e-specific T cell response induced by EcN-5M2e. Compared to EcN-vec, immunization with EcN-5M2e could significantly increase the induction of IFN-γ and IL-2 producing CD4+ T cells, but not CD8+ T cells (Fig. 4h, i; Supplementary Fig. 7). Overall, these results demonstrate that EcN-5M2e can induce M2e-specific humoral, mucosal and CD4+ T cell immune responses.
We next tested the cross-subtype reactivity of humoral and mucosal antibodies elicited by EcN-5M2e. All tested IgG or IgA antibodies of NALF, BALF or serum IgA showed similar affinity to the different subtypes of M2e peptides included in the 5M2e design (H2N2, H3N2, H5N1 and H7N9) or outside the design (H6N6, H9N2, H13N2, H17N10 and H18N11) (Fig. 4j, k). These results suggest that our EcN-5M2e vaccine has the potential to induce an effective cross-reactive immune response against a broad spectrum of influenza A viruses.
EcN-5M2e vaccination protects mice against a broad spectrum of influenza A viruses
To evaluate the protection of EcN-5M2e against influenza virus infection, immunized mice were challenged with H1N1, H3N2, or H6N6, whose M2e sequence was not included in our 5M2e design (Fig. 5a), and monitored for 14 consecutive days. Influenza virus infection caused significant weight loss during the first 9 days in mice treated with PBS or immunized with EcN-vec (Fig. 5b–d). In contrast, mice vaccinated with EcN-5M2e showed a weight loss of <10% after H1N1 or H3N2 infection and <15% after H6N6 infection on days 5–8, with gradual weight recovery in the following days (Fig. 5b–d). All mice in the PBS group succumbed to the infection before 10 days post-challenge. Conversely, all mice in the EcN-5M2e group survived after influenza virus challenge (Fig. 5e–g). The effective protection provided by EcN-5M2e against the phylogenetically distant H6N6 strain demonstrates that the EcN-5M2e vaccine provides broad protection against influenza A viruses.
a Schematic illustration of immunization, sampling and influenza A virus challenge in the mouse model. The preimmunized BALB/c mice were challenged intranasally with 10 × LD50 H1N1, 10 × LD50 H3N2 or 5 × LD50 H6N6 in 20 μL PBS. b–d Body weight of mice after challenge with a lethal dose of H1N1 (b) (PBS n = 10, EcN-vec n = 10, EcN-5M2e n = 9), H3N2 (c) (PBS n = 10, EcN-vec n = 8, EcN-5M2e n = 10), or H6N6 (d) (n = 6/group). e–g Survival of mice after challenging with lethal dose of H1N1 (e), H3N2 (f) or H6N6 (g). Panels (b–e) were representative data of 2 independent experiments. h Determination of lung virus titers at 3 or 5 dpi with H1N1 (n = 5/group). i Histopathological analysis of lung tissue sections after H1N1 infection at 5 dpi (bar represents 100 µm). Images are representative of five individual mouse lung sections in each group. Black arrows indicate thickening of the alveolar walls; Yellow arrows indicate the necrotic cellular debris; Green arrows indicate inflammatory cell infiltration; Red arrows indicate vascular blockage. j Pathological scores of lung tissue sections in mice with H1N1 infection at 5 dpi (n = 5/group). Data are presented as mean ± SEM. Statistical significance was analyzed by two-tailed unpaired t-test (panels h, j). Source data are provided as a Source Data file.
To further characterize the protection observed in the EcN-5M2e vaccination group, we examined lung virus titers and lung pathology. After challenging with H1N1, H3N2, or H6N6 viruses, BALFs were collected to measure virus titers at 3 or 5 days post infection (dpi). In contrast to the PBS group, mice vaccinated with EcN-5M2e showed a significant reduction in virus titers (Fig. 5h; Supplementary Fig. 8). Next, we performed pathological examinations of lung tissue infected with H1N1 or H3N2. Lung sections from mice immunized with PBS and EcN-vec displayed typical clinical symptoms affecting more than 50% of the lung parenchyma characterized by multifocal, moderate to severe necrotizing bronchitis and bronchiolitis, and moderate to severe alveolitis with a neutrophil-dominant, mixed inflammatory cell infiltrates and pulmonary edema. In contrast, mice immunized with EcN-5M2e did not show typical clinical symptoms (Fig. 5i, j; Supplementary Fig. 9). Collectively, these data suggest that EcN-5M2e effectively suppresses the infection of homologous (H1N1 and H3N2) and heterologous H6N6 viruses in vivo.
The protection of EcN-5M2e involves antibodies, cytokines and T cell responses
To test the protective capacity of antibodies induced by EcN-5M2e vaccination, we performed an adoptive transfer experiment. Mice were vaccinated with three doses of EcN-5M2e or controls and their sera were collected by terminal blood collection. These sera were then transferred into naïve mice by intraperitoneal administration (Fig. 6a). The IgG titers of recipients (18 h after serum transfer) were similar to those of donors (Fig. 6b), indicating successful acquisition of 5M2e antibodies. Mice were then infected with 5× LD50 of H1N1 virus 24 h after serum transfer and monitored for 14 days to assess morbidity and mortality (Fig. 6a). Mice receiving sera from donors vaccinated with EcN-vec or PBS showed severe morbidity and were not protected (Fig. 6c, d). Although body weight loss was observed in mice that received serum from the EcN-5M2e vaccinated group, about 40% of mice survived (Fig. 6c, d). These results suggest that serum antibodies induced by EcN-5M2e play a role in controlling viral infection, but are not sufficient for complete protection.
a Schematic illustration of immunization, sampling, and influenza A virus challenge in serum transfer assays. Sera from sequentially vaccinated female BALB/c mice were transferred into naïve BALB/c mice, which were then infected with 3 × LD50 of H1N1 virus. b ELISA was performed to measure endpoint titers of M2e-specific IgG in donors and recipients 18 h after transfer (n = 7/group). c Body weight of mice after a lethal dose of challenge with H1N1 (n = 7/group). d Survival of mice after a lethal dose of challenge with H1N1. e Schematic illustration of immunization, sampling and influenza A virus challenge in the mouse model. f, g Mucosal IgA levels of BALF (f) (n = 5/group) and serum (g) (n = 5/group) samples collected from vaccinated mice infected with H1N1 at 3 or 5 dpi. The absorbance at 450 nm (Abs450) in the ELISA assay against HA protein was recorded. h Heatmap display of cytokine levels in BALF after H1N1 infection at 3 dpi (n = 5/group). i, k Assessment of cytokine levels IFN-γ (i), MCP-1 (j), and TNF-α (k) in BALF samples collected at 3 or 5 dpi (n = 5/group). Data are presented as mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparison test (f, g) or two-tailed unpaired t-test (i–k). Source data are provided as a Source Data file.
The limited effect of serum M2e-specific IgG in protecting against influenza virus infection suggests that mucosal immune IgA may play an important role. In addition to M2e-specific IgA stimulation, we also tested whether the EcN-based vaccine could induce general host IgA responses against influenza virus infection. We first treated mice with PBS, EcN-vec, or EcN-5M2e, and then infected them with H1N1 (Fig. 6e). Although neither EcN-vec nor EcN-5M2e expresses HA protein, HA-specific IgA levels were higher in the BAFL of H1N1-infected mice at 3 or 5 dpi in these two groups (Fig. 6f). Serum IgA levels in these groups were similar at 3 and 5 dpi (Fig. 6g). These results suggest that vaccination with EcN-vec or EcN-5M2e facilitates host IgA responses to influenza virus infection.
Influenza virus infection can induce significant increase in pro-inflammatory cytokines, which would lead to a cytokine storm and subsequent organ damage, including lung infections, edema, and respiratory complications37. To examine the release of representative cytokines in the lung during viral infection, we collected BALF samples from mice infected with H1N1 or H3N2 viruses at 3 or 5 dpi. After 3 days of H1N1 exposure, we observed three different patterns of cytokine responses (Fig. 6h). Typically, EcN-5M2e treatment resulted in higher levels of IFN-γ (type II interferon) and IL-10 (a negative regulator of the inflammatory response) production (Fig. 6i; Supplementary Fig. 10a), which is in consistent with previous reports that IFN-γ and IL-10 play key roles in the control of influenza virus infection38,39. The levels of chemotactic cytokines, including GM-CSF, MCP-1 (CCL2), RENTES (CCL5), and KC (CXCL1), were significantly decreased in both EcN-5M2e and EcN-vec groups (Fig. 6j; Supplementary Fig. 10a), suggesting a possible role of these chemotactic cytokines in regulating infection. Type I interferons (IFN-α and IFN-β) as well as pro-inflammatory cytokines (TNF-α, IL-1β, IL-12p70 and IL-6) were all decreased in the 5M2e group (Fig. 6k; Supplementary Fig. 10a), which correlate with virus titer and lung pathology (Fig. 5h, i). These results were further confirmed in mice exposed to H3N2 (Supplementary Fig. 10b). Taken together, these data suggest that cytokines regulated by EcN-5M2e may contribute to infection control.
To elucidate the role of M2e-specific CD4+ T cells induced by EcN-5M2e in combating influenza virus, we depleted CD4+ T cells from EcN-5M2e immunized mice by injection of anti-CD4 antibody (Fig. 7a). As expected, the frequency of residual CD4+ T cells in spleen and lung was <1% in the depleted group compared with others (Fig.7b, c). When challenged with 10 × LD50 of H1N1 virus, CD4+ T cell-depleted mice exhibited greater weight loss over immunized groups without depletion or injected with isotype antibody, but retained partial protection (Fig. 7d, e). These results suggest that EcN-5M2e confers immune protection against influenza infection, at least in part, through CD4+ T cell responses.
a Schematic illustration of CD4+ T cell depletion in EcN-5M2e immunization, sampling and influenza A virus challenge in the mouse model. At 2 weeks after the second boost immunization, EcN-5M2e immunized mice were injected intraperitoneally with anti-mouse CD4 monoclonal antibody (anti-CD4) or rat IgG2a isotype control (ck) on day -3, -2, -1 and 2. On day 0, spleens and lungs were harvested and analyzed by flow cytometry to determine the efficiency of CD4+ T cell depletion. b FCM assessment of the frequency of CD4+ T cells. c Quantification of CD4+ T cells in different groups. Each dot represents one mouse (PBS: n = 5; EcN-5M2e: n = 5; EcN-5M2e+anti-CD4: n = 4; EcN-5M2e+ck: n = 3). Statistical significance was analyzed by two-tailed unpaired t-test. d, e Body weight changes (d) and survival rates (e) of EcN-5M2e-immunized mice after challenged with 10 × LD50 H1N1 influenza viruses in CD4+ T cell depletion assay (PBS: n = 6; EcN-5M2e, EcN-5M2e + anti-CD4, EcN-5M2e + ck: n = 8). Data are presented as mean ± SEM. Source data are provided as a Source Data file.
Long-lasting protection from the EcN-5M2e vaccine
The duration of the immune response is a critical factor in assessing the effectiveness of a vaccine. To evaluate the durability of the EcN-5M2e vaccine, we collected blood and mucosal samples (NALF and BALF) from mice at specific time points (Fig. 8a). Notably, the levels of M2e-specific IgG titers were only reduced 10-fold and remained above 103 180 days after the last immunization (Fig. 8b). Similarly, the levels of IgA antibodies in serum samples as well as IgA and IgG antibodies in BALF samples from the EcN-5M2e group remained consistently high 180 days after the last immunization (Fig. 8c, d; Supplementary Fig. 11a). IgA and IgG levels in NALF samples remained relatively high in the first 90 days and were still higher than in the PBS control group after 180 days (Fig. 8e; Supplementary Fig. 11b). 180 days after the 2nd booster vaccination, the mice were then infected with a lethal dose of H1N1 virus (5 × LD50). Mice that received PBS suffered significant weight loss and all died within 9 days. However, all mice immunized with EcN-5M2e survived, although they required more time to recover to the initial body weight due to the reduced antigen-specific immune responses (Fig. 8f, g). These data demonstrate that EcN-5M2e is an effective mucosal vaccine that continues to provide significant protection against fatal influenza infection even 6 months after the last immunization.
a Schematic illustration of the immunization, sampling, and experimental procedure. b The endpoint titer of IgG against H1N1-M2e peptide was measured in sera collected at time points 1, 2, 3, 4, 5 and 6 months after the 2nd booster vaccination (n = 8/group). c–e IgA at Abs450 against H1N1-M2e peptide was measured in immune sera (c), BALF (d), or NALF (e) collected at time points 1, 3, and 6 months after the 2nd booster vaccination (PBS: n = 3; EcN-5M2e: n = 5). f, g Mice vaccinated with EcN-5M2e or PBS for 6 months were infected with 5 × LD50 of H1N1 virus, body weight (f) and survival (g) were monitored for 14 days (n = 8/group). Data are presented as mean ± SEM. Statistical significance was analyzed by two-tailed unpaired t-test (panels b–e). Source data are provided as a Source Data file.
Discussion
The urgent need for novel therapeutic and preventive strategies against unanticipated epidemic or potential pandemic influenza virus strains is evident. In this study, we present convincing evidence that intranasal administration of the probiotic EcN activates host defense immunity in the lung, producing an immediate protective effect against influenza virus infection. Building on these findings, we developed a live probiotic vaccine called EcN-5M2e that expresses and secretes influenza virus antigens. The engineered EcN-5M2e triggers antigen-specific humoral and mucosal immune responses and provides broad and durable protection against influenza A viruses.
Our work here provides the first evidence that intranasal prophylaxis with EcN modulates the lung immune microenvironment and provides immediate protection against influenza virus infection in mice. Previous studies have highlighted the importance of activation of type I interferons (IFN-α and IFN-β) for the protective effects of probiotics against influenza virus infection, including Lactobacillus40,41 and Bifidobacterium42,43 species. Interestingly, administration of EcN not only significantly induced the magnitude of type I interferon response but also increased the level of type II interferon (IFN-γ) in lung tissue (Fig. 1g), which can broadly induce the expression of interferon-stimulated antiviral genes44. These results suggest that prophylactic exposure to EcN in situ may induce IFN-γ to regulate innate immune responses and promote an antiviral response.
Given the continuous antigenic drift and shift of influenza viruses, the development of a universal influenza vaccine is of utmost importance5. To address this issue, we developed a live-vector probiotic vaccine containing the 5M2e target to achieve broad-spectrum protection. Although antibodies against M2e are non-neutralizing compared to HA protein10,11, M2e-specific antibodies can bind to the M2e expressed on virus-infected host cells and reduce virus replication either by interfering with virus budding or by mediating the killing of infected lung epithelial cells by ADCC and ADCP12,45. In spite of the fact that we did not present recovered viral load data for adoptive transfer and the long-term protection experiments, our vaccine is successful in reducing lung viral load for H1N1, H3N2 and H6N6 at 3 or 5 dpi after vaccination (Fig. 5h; Supplementary Fig. 8), suggesting that EcN-5M2e is effective in suppressing influenza virus infection. Importantly, our engineered EcN-5M2e vaccine could be administered intranasally and elicits broad, potent and durable humoral and mucosal immune responses, which may provide protective effects against influenza virus infection from multiple perspectives.
Improving the magnitude and breadth of humoral immune responses is critical to the development of highly effective and broadly protective influenza vaccines46. Our results suggest that EcN-5M2e vaccination is systematically effective in enhancing antibody responses (Fig. 4). While BALB/c mice are known to typically exhibit a Th2-type immune response, which is characterized by the production of IgG1 antibodies47, it is crucial to note that a Th1-type response, distinguished by the production of IgG2a antibodies and the recruitment of cytotoxic T lymphocytes, macrophages and NK cells48,49, plays a vital role in effectively clearing viral infections50. Previous studies have also highlighted the importance of IgG2a in M2e-mediated protective immunity7,45. Our results showed that intranasal immunization with EcN-5M2e induced significantly high titers of both M2e-specific IgG1 and IgG2a (Fig. 4c, d), indicating that the EcN-5M2e vaccine induced both Th2-biased and Th1-biased immunities. By stimulating both types of immune responses, the EcN-5M2e vaccine promises to provide broad protection against influenza viruses.
In addition to serum IgG, IgA is the primary antibody produced by mucosal immune responses and plays a key role in blocking pathogen infection at mucosal tissues16. However, the efficacy of protein vaccines administered via injection is often limited in terms of inducing mucosal immunity and providing protection against mucosal infection51. This limitation arises due to the presence of adhesive mucous layers in the nasal epithelia, which act as barriers to pathogens and potentially compromise the activation of the mucosal immune system by most injectable protein-based vaccines52. In contrast, antigen delivered by live bacterial vector offers a novel and highly versatile platform53,54. EcN, in particular, has been shown to mediate interactions with mucosal epithelial cells and adhesion to the epithelial surface28, which may promote the induction of adaptive immune responses. The presence of IgA antibodies in the mucosal lavage fluids of EcN-5M2e immunized mice may increase the amplitude and breadth of these immune responses55. Therefore, immunization with EcN-5M2e has the potential to provide both homologous and heterologous protections. Combined with the limited protective effect of serum IgG in the passive transfer challenge experiment, our results suggest that IgA induced by EcN-5M2e may be a key factor in accelerating influenza virus clearance and resisting influenza virus infection.
It is interesting to note that EcN-based vaccine enhances general IgA immune responses after viral challenge, as mice administered with EcN-vec or EcN-5M2e (neither of which expresses HA) produced higher levels of HA-IgA in lung tissue after influenza virus infection than the control group (Fig. 6f), suggesting that the EcN vehicle may activate pro IgA B cells and results in faster responses to pathogen infection. This phenotype is similar to what is observed in trained immunity. This involves the enhancement of innate immune responses triggered by certain stimuli and can provide protection against related or unrelated infections56. The development of antigen-specific resident memory B cells in the lung has also been reported to produce local IgG and IgA with enhanced variant cross-recognition and correlates with protection against reinfection in mice57. However, the detailed mechanisms for these effects of EcN need to be further investigated.
Besides humoral and mucosal immune responses, we found that immunization of BALB/c mice with EcN-5M2e could significantly stimulate IFN-γ and IL-2 secretion produced by M2e-specific CD4+ T cells (Fig. 4h), and depletion of CD4+ T cells decreased the protective effects (Fig. 7). These data are consistent with previous data that T cell activation is associated with enhanced immunological memory, viral clearance and breadth of protection58,59.
In summary, our research provides compelling evidence for the potential of developing EcN as an antiviral probiotic for the prevention of influenza virus infection. Our proof-of-concept study demonstrates that this vaccine approach could have widespread use as a “superseasonal” influenza A virus vaccine. EcN’s advantageous features, including its large carrier capacity, ease of editing, and flexible antigen presentation, position it as a promising multivalent platform to combat influenza viruses and other respiratory viruses with pandemic potential.
Methods
Mice, cells, viruses, strains, plasmids and oligos
Female BALB/c mice aged 6–8 weeks were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd, Beijing, China. The mice were bred and maintained at the Animal Center of Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS). They were randomly assigned to groups and reared under specific pathogen-free (SPF) conditions (individually ventilated caging, ambient temperature 22 ± 2 °C, humidity 50 ± 5%, 12 h light/dark cycle) with ad libitum access to food and water. The infection experiments were carried out in the Animal Biosafety Level 2 (ABSL-2) Laboratory at WIV, CAS in accordance with the WIV institutional review board guidelines for animal care and use (ethics number WIVA14202101).
Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Yeasen) at 37 °C in 5% CO2.
The mouse-adapted strains of influenza A viruses, namely A/Puerto Rico/8/34 (H1N1), A/Hong Kong/4801/14 (H3N2) and A/Duck/Hubei/5/2010 (H6N6) were propagated in 10 day-old embryonated chicken eggs at 37 °C for 48 h. After virus growth, eggs were cooled overnight at 4 °C. The allantoic fluid recovered from the chilled eggs was centrifugated (4000 g for 10 min at 4 °C) to remove cellular debris. The viruses were then divided into aliquots and stored at −80 °C. The 50% lethal dose (LD50) of the viruses was measured60.
EcN and its derivative strains were cultured in Luria Bertani (LB) medium. Cells were collected by centrifugation and the resulting cell pellets were resuspended in a protective buffer (15% v/v glycerol, 5% w/v trehalose, 2% w/v β-glucan and 10 mM MOPS, pH 7.3) at a concentration of 1010 CFU/mL and were frozen at −80 °C until further use61.
All strains and plasmids used in this study are summarized in Supplementary Table 1. Oligos are listed in Supplementary Table 2.
EcN vaccinations
Mice were administrated 10 μL of EcN (108 CFU) or PBS intranasally (i.n.) on two consecutive days, and the body weight of mice was recorded daily for 7 days. To assess the influence of EcN treatment on cytokine expression, mice were humanely euthanized on day 1, 3, 7, and 14 to collect blood and lung samples.
To evaluate the in vivo safety of EcN, routine blood tests were performed on mice. Fresh blood (50 μL) was collected from each mouse and immediately mixed with EDTA. The blood samples were then examined using a standard analyzer (Hemavet 950) to determine the number of white and red blood cells and platelets.
In vivo bioluminescence imaging and colony forming unit analyses
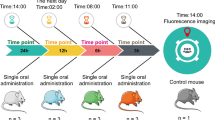
For the in vivo distribution study, mice were intranasally administered EcN carrying the pKT-mCherry plasmid (EcN-mCherry) at a dose of 108 CFU/10 μL. Fluorescence imaging of mice at specific time points was performed using an in vivo imaging system (IVIS, PerkinElmer). Multiple regions of interest (ROI) corresponding to the mouse digestive tract, nose, or lung were manually determined for each individual mouse. Fluorescence was quantified using Living Image software54. For the in vivo tissue colony forming unit analysis, tissue samples were collected at the indicated time points and mechanically homogenized in PBS containing 15% glycerol. Dilutions of the homogenates were plated on LB agar plates containing 100 μg mL-1 kanamycin and incubated overnight at 37 °C. Colony forming units (CFU) were calculated for each organ.
Phylogenetic analysis of M2e
To analyze the M2e sequences, all available full-length M2e sequences of influenza A viruses were downloaded from NCBI and duplicates were removed by Seqkit62. The resulting dataset consists of 289 unique M2e sequences derived from Homo sapiens. These sequences were aligned by Mafft63 and a maximum likelihood tree was constructed by iqtree64. The resulting tree was visualized using ggtree (accessible at http://www.bioconductor.org/packages/ggtree).
5M2e expression and purification
The 5M2e fragment was cloned into the pET21a vector using a ClonExpress II One-Step Cloning kit (Vazyme). The construct was transformed into E. coli BL21(DE3). Protein expression was induced by adding 0.3 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and incubating at 25 °C for 12 h65. The culture was centrifuged, and the pellet was resuspended in a buffer containing 40 mM Tris-HCl (pH7.9), 300 mM NaCl, 10% glycerol, and 2 mM imidazole. The cell suspension was homogenized and centrifuged at 12,000 g for 30 min at 4 °C. The supernatant was incubated with a HisTrapTM HP column (GE Healthcare), washed with a washing buffer (40 mM Tris-HCl, pH7.9, containing 300 mM NaCl, 10% glycerol, and 10 mM imidazole), and eluted with an elution buffer (40 mM Tris-HCl, pH 7.9, containing 300 mM NaCl, 10% glycerol, and 100 mM imidazole).
Recombinant EcN-5M2e construction
An EcN mutant strain lacking the dapB gene encoding dihydrodipicolinate reductase was constructed using a suicide pDM4 plasmid66. Briefly, homologous regions upstream and downstream of the dapB gene were cloned into the pDM4 plasmid, which was then transformed into E. coli S17-1 cells as a parental donor strain. EcN carrying a temperature-sensitive pKD46 plasmid67 was used as parent recipient strain in transconjugation assay61. The single cross clones were selected with ampicillin (100 μg mL−1) and chloramphenicol (30 μg mL−1), and the double cross clones were selected in LB plates containing 15% sucrose and diaminopimelate (100 μg mL−1)35. The pKD46 plasmid in EcNΔdapB was removed by culturing the strain at 42 °C67. The deletion of the dapB gene in the EcNΔdapB strain was confirmed by PCR and DNA sequencing.
To secrete the targeted 5M2e protein in the EcNΔdapB strain, the 5M2e fragment fused N-terminally to the 6×His-tag and C-terminally to the HlyA-tag (946-998 amino acids). The dapB gene, a fragment expressing type I secretion system components HlyBD68, and a P15A ori were assembled to form the pKT-5M2e-HlyABD plasmid (without antibiotic resistance gene). Next, the recombinant plasmid was transformed into EcNΔdapB to obtain a strain named EcN-5M2e, which was selected on LB medium without diaminopimelate. PCR was performed to confirm the presence of the pKT-5M2e-HlyABD plasmid in the EcN-5M2e strain. The EcNΔdapB strain transformed with the plasmid pKT-HlyABD (without 5M2e fragment) was designated as EcN-vec and used as a control.
The stability of the pKT-5M2e-HlyABD plasmid in the EcN-5M2e strain was tested. Briefly, strains were subjected to 20 passages without selective pressure. At each passage, a monoclonal colony was selected and analyzed by PCR amplification of the 5M2e fragment and the dapB-deleted region.
Growth assay of EcN-5M2e
Freshly streaked colonies of EcN WT, EcNΔdapB, EcN-vec, and EcN-5M2e were incubated overnight at 37 °C. The cultures were then transferred to fresh M9 medium at a ratio of 1:100 and incubated at 37 °C for 16 h. Hourly measurements of OD600 were taken using a BioTek instrument.
Western blot analysis
To assess the expression of the target protein, the strains were cultured overnight at 37 °C in LB medium. The cultures were centrifuged at 4000 × g for 30 min at 4 °C, and the secreted proteins in the culture supernatant were concentrated using TCA precipitation69. The proteins in the supernatant and precipitate were subjected to gel electrophoresis. The proteins in the gel were then transferred to a polyvinylidene fluoride membrane, which was blocked with 5% nonfat dry milk. The membrane was then incubated with a primary His-tag antibody (Cat#AF2876, Beyotime, 1:1000) or an M2e-specific 14C2 antibody (Cat#Ab5416, Abcam, 1:1000) for 2 h at room temperature. The membrane was then incubated with goat anti-mouse IgG conjugated to horseradish peroxidase (Cat#A0216, Beyotime, 1:10000) for 1 h at room temperature. The membrane was washed three times with TBST, incubated with Clarity Western ECL substrate (Bio-rad) to cover the blot at room temperature, and then imaged with a luminescence imager.
Mice immunization
To evaluate humoral and mucosal responses, female BALB/c mice were immunized with EcN-5M2e three times at 2 week intervals (primer-boost-boost). Each immunization was performed by intranasal administration of 108 CFU/mouse in 10 µL on two consecutive days. Control mice received the same dose of EcN-vec or the same volume of PBS. The purified 5M2e protein was immunized subcutaneously three times at an interval of 2 weeks (primer-boost-boost). One week after each immunization, serum samples were collected to detect antibody titer.
For long-term immunological evaluation, another group of mice was immunized with PBS or EcN-5M2e according to the same protocol. These mice were euthanized 1, 3 or 6 months after last immunization to collect mucosal samples. After vaccination, blood samples were collected from the orbital venous sinus at various time points and serum was isolated for antibody analysis.
Indirect immunofluorescence assay
MDCK cells were seeded into each well of a 24-well plate at a density of 2 × 105 in growth medium (DMEM supplemented with 10% fetal bovine serum) and allowed to adhere overnight. Cells were then infected with H3N2 virus and cultured for 20 h at a multiplicity of infection of 0.1, washed with PBS and fixed with 4% paraformaldehyde. The cells were then incubated with either 14C2 antibody (Abcam) at a dilution of 1:200 or sera from immunised mice at a dilution of 1:50 for 1 hour at room temperature. After three washes with PBS, Alexa Fluor® 594 anti-mouse antibody (Cat#GTX213111-05, GeneTex) was used as the secondary antibody at a dilution of 1:1000. After five washes, nuclei were stained with DAPI in the dark. Cells were observed and photographed using a fluorescence microscope (Axio Observer.A1, Zeiss).
Virus challenge
The preimmunized BALB/c mice were challenged intranasally with 10 × LD50 of H1N1, 10 × LD50 of H3N2 or 5 × LD50 of H6N6 in 20 μL of PBS. To test the protection in passive serum transfer, mice were infected with 5 × LD50 H1N1. To assess long-term protection, immunized mice were infected with 5 × LD50 H1N1. To evaluate the bacterial-induced immediate protection, mice were challenged with 2 × or 3 × LD50 H1N1. Body weight and number of mouse deaths were recorded daily until 14 dpi. Mice were humanely euthanized when their weight loss reached ≥ 30%.
Serum passive transfer experiment
Mice (donors) were primed and boosted as described above and anesthetized 2 weeks after the 2nd booster vaccination and sera were collected. Naïve BALB/c mice (recipients) were administered intraperitoneally with 300 μL of sera per mouse10. After 18 h, blood was collected from mice in each group to determine IgG titers. After 24 h, mice were infected with 5 × LD50 H1N1. The weight and survival of each mouse were monitored daily until 14 days.
Enzyme-linked immunosorbent assay
Peptide-specific IgG or IgA antibody titers in serum, nasal lavage fluid (NALF), bronchoalveolar lavage fluid (BALF), and intestinal lavage fluid (ILF) of BALB/c mice were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (Corning) were coated with 2 μg mL−1 of synthesized M2e peptides (Supplementary Table 3) or purified HA protein (H1N1) in carbonate coating buffer overnight at 4 °C and then blocked in 3% BSA in PBS for 2 h at 37 °C. The 96-well plates were incubated with serially diluted serum, NALF, BALF, or ILF samples for 2 h at 37 °C. After washing the plates 5 times with PBS containing 0.05% Tween-20 (PBST), 100 µL of HRP-conjugated goat anti-mouse IgG (Cat#A0216, Beyotime, 1:1000), IgG1 (Cat#AS066, ABclonal, 1:2000), IgG2a (Cat#AS065, ABclonal, 1:2000), or HRP-conjugated goat anti-mouse IgA (Cat#CSA2107, Cohesion Biosciences, 1:2000) was added. The plates were then incubated at 37 °C for 1 h and washed 5 times with PBST. The reactions were carried out by adding 3,3′,5,5′-tetramethytlbenzidine (TMB) for 15 min at 37 °C, and stopped with 2 M H2SO4. Absorbance at 450 nm was measured with a Synergy H1 plate reader (Bio-Tek). The endpoint titer was defined as the highest reciprocal dilution of the detected sample that resulted in an absorbance >2.1-fold of the Abs450 value of the naïve mouse samples.
Viral titration
Lung tissue was collected from mice at 3 or 5 dpi. Lungs were washed three times with sterile PBS containing 0.1% BSA. All BALF samples were stored at −80 °C. To determine viral load, MDCK cells were seeded in 96-well plates at 1 × 104 cells/well. The BALF samples were serially diluted (1:10) in DMEM (Gibco) supplemented with 2 µg mL-1 N-tosyl-L-phenylalanine chloromethyl ketone (TPCK)-trypsin (Sigma). The diluted samples were then placed into MDCK cells and incubated at 37 °C in 5% CO2 for 2 days. The neuraminidase assay was used to detect viruses in the supernatants, which were transferred to black 96-well plates and incubated with 20 μM MUNANA (Sigma) for 1 h at 37 °C. Fluorescence intensity was then measured using a Synergy H1 plate reader (Bio-Tek) with an excitation wavelength of 355 nm and an emission wavelength of 485 nm. The TCID50/mL titers were determined using the Reed-Muench method70.
Multiplex analysis of cytokines
Lungs were washed three times with PBS containing 0.1% BSA. The obtained BALF samples were stored at −80 °C. Cytokine levels were measured using the LEGENDplexTM Mouse Anti-Virus Response Panel (13-plex, BioLegend) according to the manufacturer’s recommended protocol.
Flow cytometry analysis
Flow cytometry was performed to access T cell responses in lungs after EcN treatment. Mice were euthanized and the tissues were cut into small pieces. The tissue fragments were then incubated in HBSS with 1 mg/mL collagenase I (Yeasen) and 0.1 U DNase I (Vazyme) for 30 min at 37 °C. The digested lung tissue was washed in RPMI 1640 medium, incubated in RBC lysis buffer (eBioscience) and filtered through a 70 µm cell strainer to obtain single cell suspensions. The resuspended lung cell cultures were stimulated with M2e peptide mixture (10 μg mL−1) for 16 h. Monensin (eBioscience) was added to cultures 6 h after stimulation and then incubated for a further 10 h. The cells were harvested and blocked with anti-mouse CD16/CD32 (Cat#553141, BD Biosciences) for 20 min on ice. After centrifugation, the cell suspensions were stained using surface stain antibodies together with Fixable Viability Dye eFluor™ 506 (eBioscience) for 30 min at 4 °C. The anti-mouse surface staining antibodies used to detect T cells in the lung included APC anti-mouse CD3 (Cat#155606, Biolegend), PE anti-mouse CD4 (Cat#100408, Biolegend), FITC anti-mouse CD8a (Cat#100706, Biolegend). After fixation and permeabilization with Cytofix/Cytoperm™ solution (BD Biosciences), cells were stained with combinations of the following intracellular cytokine staining antibodies: Alexa Fluor® 700 anti-mouse IL-2 (Cat#503818, Biolegend) and PE/Cyanine7 anti-mouse IFN-γ (Cat#505826, Biolegend). The gating strategy was shown in Supplementary Fig. 7. All stained cells were processed on a BD LSRFortessa and analyzed using FlowJo software.
CD4+ T cell depletion in vivo
For T cell depletion experiments, BALB/c mice were injected intraperitoneally with 200 μg of anti-mouse CD4 monoclonal antibody (clone YTS 177), or rat IgG2a isotype control (clone 2A3) at −3, −2, −1, 2 days post infection. At the day of infection, the spleens and lungs were collected and analyzed by flow cytometry to determine the efficiency of cell depletion. All antibodies were obtained from BioXCell.
Histopathology analysis
The left lung tissue was fixed in 4% paraformaldehyde for at least 48 h. After fixation, the tissues were dehydrated and embedded in paraffin71. Tissue sections with 5 µm thick were prepared and subjected to hematoxylin and eosin staining. The stained sections were then observed and photographed using a digital image analysis platform 3DHISTECH (Hungary). Two randomly selected sections of each mouse were evaluated in a double-blind manner, taking into account histopathological changes such as inflammation, structure changes and thickening of the alveolar wall. These changes were assessed according to the predefined scoring system71. To indicate lesion severity, a subjective numerical lesion severity score was assigned, ranging from 0 to 5. (0 means the lesion is absent; 1 means minimal; 2 means mild; 3 means moderate; 4 means marked; 5 means severe).
Statistics and reproducibility
The number of biological replicates and independent repeats used are described in figure legends. No statistical methods were used to predetermine sample size. No data was excluded from the analyses. Animals were randomly divided into experimental groups. Data collection and analysis were not performed blind to the conditions of the experiments. The pathologists performing the histopathological analysis were blinded to treatment. Statistical analyses were performed using GraphPad Prism 9.0 software. Values were expressed as mean ± SEM in each figure. Statistical significance for group comparisons was determined using two-tailed unpaired t-test or one-way ANOVA followed by Tukey’s multiple comparison test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available in the Article, Supplementary Information, and the Source Data file provided with this paper. Source data are provided with this paper.
References
Le Sage, V., Lowen, A. C. & Lakdawala, S. S. Block the spread: barriers to transmission of influenza viruses. Annu. Rev. Virol. 10, 347–370 (2023).
Gouma, S., Anderson, E. M. & Hensley, S. E. Challenges of making effective influenza vaccines. Annu. Rev. Virol. 7, 495–512 (2020).
Krammer, F. & Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 14, 167–182 (2015).
Feranmi, F. Universal flu vaccine protects against influenza A and B. Lancet Microbe. 3, e902 (2022).
Lo Giudice, C. Towards a universal flu vaccine. Nat. Mater. 22, 407 (2023).
Mezhenskaya, D., Isakova-Sivak, I. & Rudenko, L. M2e-based universal influenza vaccines: a historical overview and new approaches to development. J. Biomed. Sci. 26, 76 (2019).
Kavishna, R. et al. A single-shot vaccine approach for the universal influenza A vaccine candidate M2e. Proc. Natl Acad. Sci. USA 119, e2025607119 (2022).
Job, E. R. et al. Broadened immunity against influenza by vaccination with computationally designed influenza virus N1 neuraminidase constructs. NPJ Vaccines 3, 55 (2018).
Stadlbauer, D. et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366, 499–504 (2019).
Puente-Massaguer, E. et al. Chimeric hemagglutinin split vaccines elicit broadly cross-reactive antibodies and protection against group 2 influenza viruses in mice. Sci. Adv. 9, eadi4753 (2023).
Guthmiller, J. J. et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 602, 314–320 (2022).
Schepens, B., De Vlieger, D. & Saelens, X. Vaccine options for influenza: thinking small. Curr. Opin. Immunol. 53, 22–29 (2018).
Turley, C. B. et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 29, 5145–5152 (2011).
Neirynck, S. et al. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5, 1157–1163 (1999).
Deng, L. et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 9, 359 (2018).
Neutra, M. R. & Kozlowski, P. A. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6, 148–158 (2006).
Lycke, N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12, 592–605 (2012).
Thwaites, R. S. et al. Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine. Nat. Commun. 14, 8053 (2023).
Hartwell, B. L. et al. Intranasal vaccination with lipid-conjugated immunogens promotes antigen transmucosal uptake to drive mucosal and systemic immunity. Sci. Transl. Med. 14, eabn1413 (2022).
Wells, J. M. & Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6, 349–362 (2008).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Finney, S. J., Leaver, S. K., Evans, T. W. & Burke-Gaffney, A. Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med. 38, 324–332 (2012).
Vockley, J. et al. Efficacy and safety of a synthetic biotic for treatment of phenylketonuria: a phase 2 clinical trial. Nat. Metab. 5, 1685–1690 (2023).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, eaau7975 (2019).
Puurunen, M. K. et al. Safety and pharmacodynamics of an engineered E.coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat. Metab. 3, 1125–1132 (2021).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Harimoto, T. et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nat. Biotechnol. 40, 1259–1269 (2022).
Hafez, M., Hayes, K., Goldrick, M., Grencis, R. K. & Roberts, I. S. The K5 capsule of Escherichia coli strain Nissle 1917 is important in stimulating expression of toll-like receptor 5, CD14, MyD88, and TRIF together with the induction of interleukin-8 expression via the mitogen-activated protein kinase pathway in epithelial cells. Infect. Immun. 78, 2153–2162 (2010).
Hafez, M. et al. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect. Immun. 77, 2995–3003 (2009).
Le Noci, V. et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 24, 3528–3538 (2018).
Krammer, F. et al. Influenza. Nat. Rev. Dis. Primers 4, 3 (2018).
De Filette, M. et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J. Biol. Chem. 283, 11382–11387 (2008).
Tsai, H. H. et al. Lymph node follicle-targeting STING agonist nanoshells enable single-shot M2e vaccination for broad and durable influenza protection. Adv. Sci. (Weinh) 10, e2206521 (2023).
Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 16, 214–225 (2018).
Leventhal, D. S. et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 11, 2739 (2020).
Wang, C. C. et al. Airborne transmission of respiratory viruses. Science 373, eabd9149 (2021).
Flerlage, T., Boyd, D. F., Meliopoulos, V., Thomas, P. G. & Schultz-Cherry, S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 19, 425–441 (2021).
Gocher-Demske, A. M. et al. IFNgamma-induction of T(H)1-like regulatory T cells controls antiviral responses. Nat. Immunol. 24, 841–854 (2023).
Sun, J., Madan, R., Karp, C. L. & Braciale, T. J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15, 277–284 (2009).
Kumova, O. K. et al. Lung transcriptional unresponsiveness and loss of early influenza virus control in infected neonates is prevented by intranasal Lactobacillus rhamnosus GG. PLoS Pathog 15, e1008072 (2019).
Kim, S. et al. Newly isolated Lactobacillus paracasei strain modulates lung immunity and improves the capacity to cope with influenza virus infection. Microbiome 11, 260 (2023).
Groeger, D. et al. Intranasal Bifidobacterium longum protects against viral-induced lung inflammation and injury in a murine model of lethal influenza infection. EBioMedicine 60, 102981 (2020).
Zhang, Q. et al. Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol. 21, 99 (2020).
Rusinova, I. et al. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 41, D1040–D1046 (2013).
Van den Hoecke, S. et al. Hierarchical and redundant roles of activating FcgammaRs in protection against influenza disease by M2e-specific IgG1 and IgG2a antibodies. J. Virol. 91, e02500–e02516 (2017).
Maamary, J., Wang, T. T., Tan, G. S., Palese, P. & Ravetch, J. V. Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc. Natl Acad. Sci. USA 114, 10172–10177 (2017).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Miyauchi, K. et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 17, 1447–1458 (2016).
Gunay, G. et al. Peptide aggregation induced immunogenic rupture (PAIIR). Adv. Sci. (Weinh) 9, e2105868 (2022).
Constant, S. L. & Bottomly, K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15, 297–322 (1997).
Lamm, M. E. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51, 311–340 (1997).
Wang, S., Liu, H., Zhang, X. & Qian, F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell 6, 480–503 (2015).
Medina, E. & Guzman, C. A. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 19, 1573–1580 (2001).
Sarate, P. J. et al. E. coli Nissle 1917 is a safe mucosal delivery vector for a birch-grass pollen chimera to prevent allergic poly-sensitization. Mucosal. Immunol. 12, 132–144 (2019).
Okuya, K. et al. Potential role of nonneutralizing IgA antibodies in cross-protective immunity against influenza A viruses of multiple hemagglutinin subtypes. J. Virol. 94, e00408–e00420 (2020).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Onodera, T. et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl Acad. Sci. USA 109, 2485–2490 (2012).
Wilkinson, T. M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Eliasson, D. G. et al. M2e-tetramer-specific memory CD4 T cells are broadly protective against influenza infection. Mucosal. Immunol. 11, 273–289 (2018).
Xu, L. et al. Adaption of seasonal H1N1 influenza virus in mice. PLoS One 6, e28901 (2011).
He, L. et al. Rational design of a genome-based insulated system in Escherichia coli facilitates heterologous uricase expression for hyperuricemia treatment. Bioeng. Transl. Med. 8, e10449 (2023).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: A cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One 11, e0163962 (2016).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Shi, W. et al. Structural basis of bacterial sigma(28) -mediated transcription reveals roles of the RNA polymerase zinc-binding domain. EMBO J. 39, e104389 (2020).
Milton, D. L., O’Toole, R., Horstedt, P. & Wolf-Watz, H. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178, 1310–1319 (1996).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Zhao, H., Lee, J. & Chen, J. The hemolysin A secretion system is a multi-engine pump containing three ABC transporters. Cell 185, 3329–3340.e3313 (2022).
Flaugnatti, N. & Journet, L. Identification of effectors: precipitation of supernatant material. Methods Mol. Biol. 1615, 459–464 (2017).
Lei, C., Yang, J., Hu, J. & Sun, X. On the calculation of TCID(50) for quantitation of virus infectivity. Virol Sin. 36, 141–144 (2021).
Dong, C. et al. Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo- and heterologous strains. Proc. Natl Acad. Sci. USA 118, e2024998118 (2021).
Acknowledgements
We thank the National Virus Resource Center for providing strains A/Puerto Rico/8/34 (H1N1), A/Hong Kong/4801/14 (H3N2), and A/Duck/Hubei/5/2010 (H6N6), and Dr. Jianjun Chen for providing HA protein for H1N1.We also extend our thanks to Juan Min from the Center for Instrumental Analysis and Metrology at Wuhan Institute of Virology for technical support in flow cytometry analyses, and to the Center for Animal Experiment at Wuhan Institute of Virology for assistance with the animal experiments. This work was supported by the CAS Interdisciplinary Innovation Team (to Y.H.) and the Young Top-notch Talent Cultivation Program of Hubei Province (to Y.H.).
Author information
Authors and Affiliations
Contributions
S.C. and Y.H. conceived the project. Ling H., W.T., Lina H., S.C. and Y.H. designed the experiments. Ling H and Lina H constructed the EcN-5M2e strain and the antigen delivery system. Ling H., W.T., Lina H., M.L. and Zhouyu W. performed animal experiments. Zhiyong W. performed bioinformatics analyses. Ling H., W.T., Lina H., X.L, A.H., X.H., S.C. and Y.H. analyzed data. Ling H. and Y.H. wrote the manuscript with input from W.T., A.H., X.L., X.H. and S.C.
Corresponding authors
Ethics declarations
Competing interests
Ling H., W.T., S.C., and Y.H. have filed patents related to the EcN-5M2e vaccine described in this manuscript. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, L., Tang, W., He, L. et al. Engineered probiotic Escherichia coli elicits immediate and long-term protection against influenza A virus in mice. Nat Commun 15, 6802 (2024). https://doi.org/10.1038/s41467-024-51182-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51182-3
- Springer Nature Limited