Abstract
Vaccine responsiveness is often reduced in older adults. Yet, our lack of understanding of low vaccine responsiveness hampers the development of effective vaccination strategies to reduce the impact of infectious diseases in the ageing population. Young-adult (25–49 y), middle-aged (50-64 y) and older-adult ( ≥ 65 y) participants of the VITAL clinical trials (n = 315, age-range: 28-98 y), were vaccinated with an annual (2019–2020) quadrivalent influenza (QIV) booster vaccine, followed by a primary 13-valent pneumococcal-conjugate (PCV13) vaccine (summer/autumn 2020) and a primary series of two SARS-CoV-2 mRNA-1273 vaccines (spring 2021). This unique setup allowed investigation of humoral responsiveness towards multiple vaccines within the same individuals over the adult age-range. Booster QIV vaccination induced comparable H3N2 hemagglutination inhibition (HI) titers in all age groups, whereas primary PCV13 and mRNA-1273 vaccination induced lower antibody concentrations in older as compared to younger adults (primary endpoint). The persistence of humoral responses, towards the 6 months timepoint, was shorter in older adults for all vaccines (secondary endpoint). Interestingly, highly variable vaccine responder profiles overarching multiple vaccines were observed. Yet, approximately 10% of participants, mainly comprising of older male adults, were classified as low responders to multiple vaccines. This study aids the identification of risk groups for low vaccine responsiveness and hence supports targeted vaccination strategies. Trial number: NL69701.041.19, EudraCT: 2019-000836-24.
Similar content being viewed by others
Introduction
Vaccination is crucial in our fight against morbidity and mortality caused by infectious diseases. Yet, some individuals, specifically those of older age are not sufficiently protected by today’s vaccination programs, which entails risks for severe disease. These gaps in protection lead to high medical costs and increased societal impact of infectious diseases in the rapidly aging population1,2,3. Hence, more effective vaccination strategies for older adults are urgently needed.
Currently, annual influenza vaccination as well as vaccination against pneumococci is advised for adults above 65 years of age in most countries. In the Netherlands, both vaccines are advised from the age of 60 years old. Likewise, older individuals have been a focus group for vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, vaccination efficacy as well as humoral and cellular immune responses decline with advancing age4,5,6,7,8,9. This decline in vaccine-induced immunity is a result of a general functional deterioration of the immune system with advancing age, also referred to as immunosenescence10,11,12,13, and may be associated with sex hormones14,15. Previous research has unraveled phenotypical changes in the aging immune system and has identified major influences for non-heritable factors in aging immunity16. Still, this has not yet led to a better understanding of reduced vaccine responsiveness.
Remarkably, vaccine responsiveness is highly variable among individuals, indicating that the pace of immunosenescence varies between individuals17,18. Subsequently, future successful vaccination strategies for the aging population could be directed more towards groups or individuals most at risk for severe infections and low vaccine responsiveness, rather than chronological age only.
The development of new vaccination strategies for these groups is currently hampered by our lack of understanding of the immunological mechanisms underlying inferior responses to vaccination. Moreover, head-to-head comparison of an individuals’ responses towards multiple vaccines is required to determine whether the risk for low vaccine responsiveness depends on vaccine type or is transcending over multiple vaccines. To identify risk groups for low vaccine responsiveness, associations of demographic characteristics and health status with low vaccine responsiveness should be thoroughly investigated. The age-associated decline in health, leading to physical impairment, disease, and mortality could be captured in the Frailty Index19,20.
To increase our knowledge on vaccine responsiveness with advancing age, we here present the results of an unique vaccination study, in which individuals divided over 3 age groups (young adults (YA) 25–49 y, middle-aged adults (MA) 50–64 y, older adults (OA) ≥ 65 y) were consecutively vaccinated with 3 different vaccines within a timeframe of two years21. Every participant received a seasonal quadrivalent inactivated influenza (QIV) booster vaccination in the autumn of 2019, followed by a primary 13-valent pneumococcal-conjugate (PVC13) vaccination up to a year later (summer/autumn 2020), and finally a primary vaccination series of two SARS-CoV-2 mRNA-1273 (mRNA-1273) vaccines in the spring of 2021.
The primary aim of the study was to compare the short-term (28 days) humoral vaccine responses following primary (PCV13 and mRNA-1273) and booster (QIV) vaccination between the different age groups, whereas the secondary aim was to compare the persistence of these humoral responses 6 months post-vaccination between the age groups. Finally, this study has the unique opportunity to explore vaccine responsiveness transcending over multiple different vaccines within the same individual and associate responsiveness to health demographics such as the Frailty Index.
Our results indicate comparable induction of humoral immunity following booster QIV vaccination in all age groups, whereas lower humoral responses following primary PCV13 and mRNA-1273 vaccinations were observed in older adults. Secondly, a shorter persistence of humoral responses following all vaccines was observed in the older adults. Importantly, we show that the quantity of vaccine-induced humoral immunity within one individual is vaccine-type specific. However, a small group of primarily older male adults showed low antibody concentrations following multiple vaccines. This finding guides the identification of risk groups for low vaccine responsiveness and supports targeted vaccination strategies.
Results
Study population
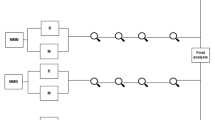
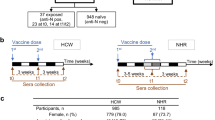
A total of 326 participants were included in this study (Fig. 1A), of whom 315 participants met the per-protocol (PP) criteria for at least one vaccine (YA n = 59, MA n = 95, OA n = 161) (Fig. 1B; Supplementary Table 1). The average age of young, middle-aged and older adults was 36 (range 25–49), 58 (range 50–64), and 76 (range 65–98) years respectively. 34%, 41%, and 53% of young, middle-aged, and older adults respectively were male. The mean body mass index (BMI) ranged from 24 in the young to 26 in the older adults. Middle-aged and older adults on average received 5 seasonal influenza vaccinations since 2014, whereas this was on average 3 in the young adults. The median frailty index increased from 0.07 (range 0.0–0.27) in the young adults to 0.10 (range 0.01–0.36) in the middle-aged and 0.18 (range 0.03–0.53) in older adults. As expected, the number of medications and incidence of chronic health conditions were higher in the older age groups compared to the young adults.
A Timeline of vaccination cohort with blood drawing timepoints from which samples are used in this reported indicated. The samples used for the primary endpoint analysis (per vaccine) are indicated in bold and underlined. (#) Pre-QIV blood sampling started in summer 2019, while vaccination started in autumn 2019, when the vaccine was available. (*) The SARS-CoV-2 pandemic lockdown caused a temporary interruption of the trial. N = 40 participants were sampled before the lockdown. For the other participants, the 6 months post-QIV vaccination timepoint was extended up to 12 months. Sampling and PCV13 vaccination started again in summer/autumn 2020. Created with Biorender.com (B) Participants flow chart indicating the number and age of participants included in the primary endpoint analyses of the different arms of the cohort. Exclusions are divided into definite and temporarily exclusions and reasons for exclusion are given. (*) A large group of older participants was excluded for mRNA-1273 vaccination, due to administration of prior COVID-19 vaccination in the general vaccination program of the Netherlands in winter 2021. This group is analyzed separately as BNT162b2 study group.
The primary endpoint of the study, humoral response 28 days post-vaccination, was analyzed in 307 participants (YA n = 56, MA n = 94, OA n = 157) for the QIV vaccine, 275 participants (YA n = 51, MA n = 84, OA n = 140) for the PCV13 vaccine and 202 participants (YA n = 43, MA n = 75, OA n = 84) for the mRNA-1273 vaccine. Importantly, 43 older individuals (mean age = 84 y (range 76–98 years)) received the BNT126b2 vaccine during the regular COVID-19 vaccination program in the Netherlands and hence were analyzed separately.
Additionally, humoral responses for both QIV and PCV13 vaccination were available and analyzed in 267 participants (YA n = 48, MA n = 83, OA n = 136) while 190 participants (YA n = 41, MA n = 70, OA n = 79) had data available for all three vaccines, including mRNA-1273. The numbers and reasons for exclusions in every step of the clinical trial are depicted on the left side of Fig. 1B. Of note, some participants were temporally excluded from the QIV (n = 8) and PCV13 (n = 11) analysis due to missing titer information or short-term corticosteroid use. Baseline characteristics for all different sub-analyses of the clinical trial are depicted in Supplementary Table 1.
Booster QIV vaccination induced protective H3N2-specific HI titers in the majority of individuals from all age groups
QIV responsiveness was analyzed using the influenza A-specific hemagglutination inhibition (HI) titers. The annual booster QIV vaccination induced a significant (p < 0.0001) increase in H3N2 HI titer 28 days post-QIV vaccination in all age groups compared to the pre-QIV timepoint (Fig. 2A). The percentage of H3N2-specific responders, based on an HI titer of ≥40 at 28 days post-QIV vaccination and an increase of >4 as compared to the pre-QIV timepoint, was 61%, 66% and 60% in the young adults, middle-aged adults and older adults respectively. At this timepoint, the H3N2-specific HI titers did not significantly differ between the age groups (median (min-max)): (YA = 136.5 (5–1810), MA = 80.0 (5–1280), and OA = 80.0 (5–1810)) (Fig. 2A). Moreover, no correlation (r = −0.011, р = 0.85) between age and H3N2-specific HI titers 28 days post-vaccination was observed (Supplementary Fig. 1A). Of note, a slightly higher H3N2 titer was observed in female older adults as compared to male older adults (p = 0.012) (Supplementary Table 2). However, these differences were not significant after correcting for multiple testing. A positive correlation between the pre-vaccination and 28 days post-vaccination H3N2 titers was observed in all age groups (YA: r = 0.444 p = 0.0006, MA: r = 0.487 p < 0.0001, OA: r = 0.527 p < 0.0001 (Supplementary Fig. 1B)).
The pre-QIV and 28 days post-QIV vaccination H3N2 (A) and H1N1 (B) specific HI GMT titers split by age group (young adults n = 56, middle-aged adults n = 94, older adults n = 157). The boxplots indicate the median and interquartile range. All individual values, from min to max, are plotted behind the boxplot. The pre- and post-vaccination timepoints were compared between the different age groups with the Kruskall–Wallis test and corrected for multiple comparisons with Bonferroni correction. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The exact p-values at the 28 days post-vaccination time are: YA vs MA: p-value = 0.002, YA vs OA: p-value < 0.0001. C The long-term H3N2-specific HI titers per age groups plotted at the timepoint of sampling. D The longitudinal H3N2 HI-specific titers. The lines indicate the mean and standard error of the mean. In both (C and D) n = 52 young adults, n = 87 middle-aged adults, and n = 142 older adults were included and for clarity reasons, no statistics are indicated in these graphs. The gray dotted lines indicate an HI titer of 40, the cut-off level for protection. Source data are provided as a Source data file.
Whereas high pre-vaccination H1N1 HI titers were observed in all age groups, these titers were significantly higher in young adults as compared to the other age groups (MA: р = 0.0003, OA: р < 0.0001). Similarly, 28 days post-QIV vaccination the H1N1 HI titers were significantly higher in young adults as compared to middle-aged (p = 0.002) and older adults (p < 0.0001) (median (min-max)): YA:160 (5–905.1), MA:68.3 (5–640), and (OA:56.6 (5–2560)) (Fig. 2B). However, based on the above-mentioned responder criteria, only 5%, 16% and 13% of the young adults, middle-aged adults and older adults respectively, were classified as responder for H1N1, which can be explained by the high pre-vaccination titers in this cohort. Therefore, influenza A-specific responsiveness following QIV vaccination was further studied using the H3N2-specific HI titers.
Persistence of the humoral response was assessed in 281 participants (52 young adults, 87 middle-aged adults, and 142 older adults). Due to the COVID-19 lockdown the majority of the samples was drawn around 300 days post-QIV vaccination in all age groups (Fig. 2C). Despite this enlarged sampling window, the distribution of sampling time was equal between the age groups and allowed for longitudinal analysis (Fig. 2D). Mixed model analysis revealed a significantly lower H3N2 HI titer at this long-term timepoint in both middle-aged (β Coeff. [std error] = −0.60 [0.23] p = 0.01) and older adults (β Coeff. [std error] = −0.50 [0.21] p = 0.018) as compared to young adults (predicted values [95%CI]: YA = 77.93 [54.53–111.37], MA = 42.97 [32.61–56.63] and OA = 47.0 [37.87–58.33]).
While not significantly different (p = 0.515), middle-aged and older adults show proportionally more participants with an H3N2 HI titer below 40 (YA = 28.3%, MA = 36.8%, and OA = 36.6%) at this long-term timepoint (Fig. 2C). More importantly, 3.8% of the young, 13.8% of the middle-aged, and 16.2% of the older adults possessed H3N2 titers under the assay detection limit of 10. Also at this long-term timepoint, a higher H3N2 titer was observed in female as compared to male older adults (p = 0.04)(Supplementary Table 2).
Primary PCV13 vaccination induced lower IgG responses and a limited breadth of response in older adults
Next, we analyzed the pneumococcal serotype-specific antibodies induced by the primary PCV13 vaccination. A significant increase in IgG concentrations at the 28 days post-vaccination timepoint was observed for all 13 serotypes in all age groups (p < 0.0001 for all) (Fig. 3A). Pre-vaccination, older adults had significantly lower IgG concentrations for serotypes 1 (YA: p = 0.001, MA: p = 0.035) and 3 (YA: p = 0.0004, MA: p < 0.0001), whereas middle-aged adults showed significantly higher serotype 6B (OA: p = 0.013) and 14 (YA: p = 0.004, OA: p = 0.018) specific IgG as compared to the other age groups (Fig. 3A).
A The pre- and 28 days post- PCV13 vaccination pneumococcal serotype-specific IgG concentrations (ug/mL) (presented on a log10 scale) split by age group (young adults n = 51, middle-aged adults n = 84, older adults n = 140). The boxplots indicate the median and interquartile range. All individual values, from min to max, are plotted behind the boxplot. Per timepoint, the antibody concentrations are statistically compared between the 3 different age groups with the Kruskall–Wallis test and corrected for multiple comparisons with Bonferroni correction. The exact p-values at the 28 days post-vaccination timepoint comparing YA and OA: serotype 1: p = 0.008, 4: p < 0.0001, 6A: p = 0.007, 6B: p = 0.0002, 23 F: p = 0.0001 and comparing YA and MA: serotype 4: p = 0.002, 23 F: p = 0.01. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. B Comparison of the number of pneumococcal serotypes an individual is responding to 28 days post-PCV13 vaccination based on a serotype-specific IgG concentration of ≥1.3 µg/mL and an at least 2-fold increase as compared to the pre-vaccination timepoint between the different age groups (young adults n = 51, middle-aged adults n = 84, older adults n = 140). The black lines indicate the median. The different age groups are compared with the Kruskall–Wallis test and corrected for multiple comparisons with Bonferroni correction. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The exact p-values are: MA vs YA: p = 0.027, OA vs YA: p < 0.0001. C The longitudinal (pre-, 7 days, 28 days, and 6 months post PCV12 vaccination) pneumococcal strain-specific IgG concentrations split by the different age groups (young adults n = 45, middle-aged adults n = 78, and older adults n = 134). The lines indicate the mean and standard error of the mean. Source data are provided as a Source data file.
At 28 days post-PCV13 vaccination, the IgG concentrations specific for serotypes 1, 4, 6A, 6B, and 23F, were found significantly lower in older adults as compared to the young adult (serotype 1: p = 0.008, 4: p < 0.0001, 6A: p = 0.007, 6B: p = 0.0002, 23F: p = 0.0001), whereas middle-aged adults also showed a significantly lower serotype 4 and 23F-specific IgG concentration as compared to the young adults (serotype 4: p = 0.002, 23F: p = 0.01) (Fig. 3A). A trend towards a lower IgG concentration at the 28 days post- PCV13 vaccination timepoint was also observed for the remaining serotypes (Supplementary Table 3). Weak but significant negative correlations between age and the 28 days post-PCV13 IgG concentrations were observed for the pneumococcal serotypes 1 (r = −0.156, p = 0.009),4 (r = −0.235, p < 0.0001),5 (r = −0.183, p = 0.002),6A (r = −0.146, p = 0.015),6B (r = −0.208, p = 0.001), and 23F (r = −0.251, p < 0.0001)(Supplementary Table 4).
In addition, positive correlations were observed between the pneumococcal serotype-specific pre-vaccination and 28 days post-PCV13 vaccination IgG concentrations for the majority of serotypes, the strength of these correlations was similar in all age groups (Supplementary Fig. 2). Moreover, the IgG concentrations specific for serotypes 5, 6A and 6B strongly correlated amongst each other in all age groups. Interestingly, the 28 days post-PCV13 vaccination IgG concentrations for the remaining serotypes did not or only weakly correlate within the young adults, while a stronger correlation was observed in the middle-aged and especially older adults (Supplementary Fig. 3).
Subsequently, the breadth of the response to PCV13 was investigated. Here the number of serotypes an individual is responding to was calculated, based on a serotype-specific IgG concentration ≥1.3 ug/mL at 28 days post-PCV13 vaccination and at least a 2-fold increase as compared to the pre-PCV13 timepoint, criteria commonly used to identify pneumococcal antibody responders22,23,24. Middle-aged (p = 0.027) and older adults (p < 0.0001) significantly responded to a lower number of serotypes as compared to young adults (Fig. 3B). Furthermore, the percentage of participants with a response to at least 70% of the serotypes, criteria also often employed to determine PCV13 responsiveness22,23,24, was significantly higher in the young adults (80%) as compared to middle-aged (58%, p = 0.0084) and older adults (48%, p < 0.0001). Specifically, the percentage of older adults responding to the serotypes 1 (p = 0.008), 4 (p = 0.0001), 6A (p = 0.01), 6B (p = 0.004), 7F (p = 0.04), 9V (p = 0.002) and 23F (p = 0.0001) was significantly lower as compared to the young adults and for serotypes 4 (p = 0.04), 7F (p = 0.02), 9V (p = 0.02) and 23F (p = 0.03) as compared to the middle-aged adults, whereas the percentage of middle-aged adults responding to serotypes 1 (p = 0.03), 4 (p = 0.03) and 23F (p = 0.03) was significantly lower as in the young adults (Supplementary Table 3). Combined these results emphasize a gradual decline in the breadth of the response to PCV13 from young to older adults.
Interestingly, the variation in IgG concentrations was significantly different between the age groups for the serotypes 3 (p = 0.022), 4 (p < 0.001), 7 F (p = 0.098), 9V (p < 0.001), 19F (p = 0.043) and 23 F (p = 0.03), serotypes for which older adults showed a large variation in antibody concentrations. Hence, the longitudinal response to PCV13 vaccination (pre-PCV13 and 7 days, 28 days, and 6 months post-PCV13) in 45 young adults, 78 middle-aged adults, and 134 older adults (Fig. 3C) was analyzed using linear mixed models. This analysis revealed significantly lower IgG concentrations to all serotypes, except to serotype 14, 7 days post-vaccination in older adults as compared to younger adults, whereas this was only observed for serotypes 1, 4, and 23F in middle-aged adults (Fig. 3C and Supplementary Table 5). At 6 months post-PCV13 vaccination, serotypes 1, 3, 4, 6A, 6B, and 23F-specific IgG concentrations were lower in the older adults as compared to the young adults, which was only found for serotype 23F in middle-aged adults (Fig. 3C and Supplementary Table 5).
Of interest, no associations between sex and PCV13 responsiveness were observed (Supplementary Table 2).
A primary series of mRNA-1273 vaccination induced lower Spike-specific IgG responses in older adults
Next, we analyzed the induction of humoral responses following a primary series of 2 doses of the mRNA-1273 vaccine. A strong increase in Spike-specific S1 IgG concentration was observed in all age groups (p < 0.0001 in all) (Fig. 4A). Pre-vaccination, only 2 young, 4 middle-aged, and 4 older adults possessed a Spike-specific S1 IgG concentration above the seropositivity level of 10 BAU/mL. Moreover, a nucleocapsid (N) specific IgG concentration above the cut-off level for seropositivity (14.3 BAU/mL) was observed in only 1 young, 3 middle-aged, and 1 older-adult, which confirms that this cohort is highly naïve for SARS-COV2 before vaccination. Also, these pre-vaccination N-specific IgG concentrations did not differ between the age groups (p = 0.61) (Fig. 4B). At 28 days post 2nd mRNA-1273 vaccination, a strong trend to a lower Spike S1-specific IgG concentration was observed in older adults compared to young adults (p = 0.065) (geometric mean IgG concentration in BAU/mL (95% CI): YA = 2587.8 (2203.9–3038.5), MA = 2217.9 (1874.5–2623.3), and OA = 1832.3 (1513.5–2218.3)) (Fig. 4A). This finding was supported by the observation of a weak negative correlation between the Spike S1-specific IgG and age (r = -0.165, p = 0.02) (Supplementary Fig. 5A). Interestingly, the variation in antibody concentrations at this timepoint differed between the age groups (p = 0.024) and was enlarged in the older adults. While not remaining significantly after correction for multiple testing, the Spike-specific S1 IgG concentrations were significantly higher in female middle-aged adults as compared to middle-aged males (p = 0.02)(Supplementary Table 2).
A The pre- and 28- days post 2nd mRNA-1273 vaccination Spike S1-specific IgG concentrations (BAU/mL) (presented on a log10 scale) split by age group (young adults n = 43, middle-aged adults n = 75, older adults n = 84). The boxplots indicate the median and interquartile range. All individual values, from min to max, are plotted behind the boxplot (B) The nucleocapsid (N) specific IgG concentrations (BAU/mL) at the pre-vaccination timepoint compared between the different age groups. The dotted line indicated the N-specific cut-off for seropositivity (14.3 BAU/mL). C The longitudinal (pre-, 28 days, and 6 months post 2nd mRNA-1273 vaccination) S1-specific IgG concentrations split by the different age groups (young adults n = 41, middle-aged adults n = 73, and older adults n = 82). The lines indicate the mean and standard error of the mean. No statistics are indicated in this graph. In both (A and C), the gray dotted line (S1-specific IgG = 10 BAU/mL) indicates the cut-off for seropositivity. The black dotted line (S1-specific IgG = 300 BAU/mL) indicates the cut-off for a high response. Source data are provided as a Source data file.
Also here, the mixed models investigating the longitudinal responses indicate a significantly lower Spike S1-specific IgG concentration in the older adults as compared to the young adults (β Coeff. [std error] = −0.48 [0.17] p = 0.0071) (predicted values [95%CI]: YA = 414.3 [313.1–548.1], MA = 323.9 [262.6–399.5] and OA = 257.6 [211.3–314.0] BAU/mL) 6-month post the 2nd vaccination (Fig. 4C). At this long-term timepoint, all participants still had a Spike-specific S1 IgG concentration above the seropositivity level of 10 BAU/mL. Moreover, higher Spike-specific S1 IgG concentrations were found in middle-aged (p = 0.02) and older (p = 0.02) female participants as compared to their male counterparts (Supplementary Table 2).
Noteworthy, the oldest adults who were vaccinated with BNT162b2 in the general vaccine program (mean age 84 years, range 76–98 years) also showed an adequate (above 300 BAU/mL) Spike S1-specific IgG concentration (geometric mean 629.3 (range 462.5–1036.0) BAU/mL) 28 days post 2nd BNT162b2 vaccination (Supplementary Fig. 4A). A trend towards a significant negative association between the Spike S1-specific IgG concentrations and age was also observed in this sub-cohort (r = −0.279, p = 0.07) (Supplementary Fig. 5B). In this group, the S1-specific geometric mean IgG concentration at 6 months post 2nd BNT162b2 vaccination was reduced to 88.1 (52.1–149.2) BAU/mL (Supplementary Fig. 4B) and 3 older adults had an S1-specific IgG concentration below the seropositivity level of 10 BAU/mL.
Response profiles transcending over multiple vaccines largely vary between individuals independent of age groups, yet a small group of individuals responds low to multiple vaccines
Finally, to identify individuals at risk for general low vaccine responsiveness, an individual’s responsiveness towards multiple vaccines was investigated. Initial analysis indicated an absence of correlation between the 28 days post-QIV, PVC13, and mRNA-1273 vaccination antibody titers in all age groups (Supplementary Table 6).
To allow comparison of the response between the different vaccine-types, the 28 days post- vaccination antibody titers were divided into quartiles, and an individual’s response score was determined for all vaccines separately (1 = low, 2–3 = nominal, and 4 = high) (see “Methods” section). Initial analysis focused on the QIV and PCV13 responses in the 267 participants included in the dual vaccine analysis (Fig. 1B). The percentage of individuals in the lowest category (1) was lowest in the young adults (QIV: 14.6% and PCV13: 8.3%) and largest in the older adults (QIV: 30.1% and PCV13: 30.9%) (Supplementary Table 7). Interestingly, the proportions of individuals in the highest response category (4) was similar between the young (QIV: 27.1% and PCV13: 25%) and older adults (QIV: 25.7% and PCV13: 29.4%). Comparison of the response scores between the 2 vaccines within one individual reveals large differences in the level of induced humoral immunity between these 2 vaccines (Fig. 5A). Next, the dual vaccine response score per individual was defined as the average quartile score for QIV and PCV13 and demographic characteristics were compared between individuals in the 4 different dual response groups (Table 1). Individuals in the lowest dual vaccine response group (n = 20, 7.5%), our primary interest, were older as compared to the other response groups and more often of the male sex (n = 14, 70%). In addition, a lower pre-vaccination H3N2 vaccination titer was observed in the lowest dual vaccine response group, whereas BMI, the Frailty Index, EQ-5D-3L score, and the number of prescription medications used were equal between the groups.
A Dual (QIV and PCV13) and B Triple (QIV, PCV13, and mRNA-1273) vaccine responsiveness. The 28 days post-vaccination antibody titers are divided into quartiles, where number 1 indicated the lowest and number 4 the highest quartile of responders. Per vaccine, each individual is assigned to a quartile based on the 28 days post-vaccine antibody concentration. Every line indicates the trajectory of an individual between the different vaccines indicated. The line color indicated the age group of an individual. C Hierarchical clustering of all individuals based on the response scores for all three vaccines. The scores indication ranges from gray (low,1) to dark blue (high, 4). Age group, sex, and cluster number are depicted in the different colors on the left side of the heatmap. In (A): young adults n = 48, middle-aged adults n = 83, and older adults n = 136, and in (B, C) young adults n = 41, middle-aged adults n = 70, and older adults n = 79. Source data are provided as a Source data file.
Subsequent analysis of triple vaccine responsiveness (Fig. 5B) in a sub-cohort of 190 individuals (Fig. 1B) indicated a large mixture in humoral immunity induced by multiple vaccines, both within and between individuals. Due to the exclusion of a group of individuals who did not receive mRNA-1273 vaccination, quartiles in antibody concentrations slightly changed. Yet again, the individuals with the lowest triple vaccine response scores (n = 17, 8.9%, defined as the average score between the 3 vaccines), were of higher age and mainly of the male sex (n = 14, 82%), whereas frailty status and BMI did not differ (Table 1). Compared to the highest response group, the lowest responders again had significantly lower pre-vaccination H3N2 titers.
To enhance our understanding on vaccine response patterns overarching three vaccines, unsupervised hierarchical clustering on the response scores was applied. This analysis identified nine different clusters of individuals with distinct vaccine responder profiles (Fig. 5C). While cluster 4 (low to all, n = 32, 16.8%) and 7 (high to all, n = 20, 10.5%) clearly indicate the extremes of responses, all other clusters include participants with variable responses to the different vaccines. Noteworthy, this analysis indicated a slightly closer relation between the QIV and mRNA-1273 response than the PCV13 response. Age group and sex are depicted on the left side of the heatmap in Fig. 5C and demographic characteristics for all clusters in Table 2. Despite limitations in statistical power, some interesting observations can be made. Firstly, in agreement with the analyses performed above, older adults are present in all clusters. Nevertheless, the percentage of older adults varies between the clusters. Specifically, older adults make up 70% of individuals in cluster 1, the cluster that only responded high to the QIV vaccine, which is likely related to high pre-vaccination H3N2 titers. Also cluster 4 (low to all) harbors a high percentage of older adults (53.1%) and has the highest mean age (64.2 y). On the contrary, the percentage of older adults is lowest (23.1%) in cluster 6 (high response to mRNA-1273, intermediate to QIV, and low to PCV13) and the mean age is lowest (54.7 y) in cluster 7 (high response to all vaccines). Interestingly, both these clusters 4 and 7 harbor a high percentage of male participants compared to all other clusters, whereas both clusters with only low responses to PCV13 contain the lowest percentage of males (cluster 6: 30.8%, cluster 9: 32%).
Furthermore, low H3N2 pre-vaccination titers were observed in the clusters (4, 5, and 8) that respond low to QIV. No clear associations between BMI, frailty index, EQ-5D-3L, and number of medications with the different clusters were observed.
Combined these results indicate that the level of vaccine-induced humoral immunity is highly variable in all age groups, though low responsiveness is transcending over multiple vaccines for approximately 10–15% (n = 20–32) of the participants, depending on the method used. These general low responders were primarily older male adults with low pre-vaccination H3N2 HI titers.
Discussion
The primary outcomes of this unique clinical trial indicate equal peak antibody responses following an annual QIV (season 2019–2020) booster vaccination in old and young individuals, while lower humoral responses are observed following both primary PCV13 and mRNA-1273 vaccinations in older adults compared to middle-aged and young adults. Additionally, our results suggest a shorter persistence of this humoral immunity in older adults for all vaccines. Importantly, highly variable vaccine response patterns transcending over multiple vaccines were observed in all age groups, yet about 10–15% of participants responded low to all vaccines. These low responders were primarily of the male sex and had low pre-vaccination titers for influenza.
Contrary to previous indications of reduced responsiveness to annual influenza vaccination in older adults7,9,25,26,27, the 2019–2020 booster QIV vaccine administered in this cohort induced statistically equal H3N2-specific HI responses 28 days post-vaccination in young, middle-aged, and older adults, including an equal proportion of responders. One explanation for this could be potential differences in immunogenicity between seasonal influenza vaccinations and strains. Moreover, most existing studies on aging and influenza vaccine responsiveness derive from cohorts that exhibit substantial differences in pre-vaccination immunity between younger and older individuals. By ensuring the administration of a booster vaccination in all age groups, based on the inclusion of previously influenza-vaccinated individuals in 2018–2019, the current study partly mitigates the impact of pre-vaccination differences in the analysis of QIV responsiveness. As also previously noted by others28, low H1N1-specific HI responses were observed after the QIV vaccination, which can be explained by high pre-vaccination H1N1-specific HI titers. We suggest that limited antigenic drift between H1N1 vaccine strains in previous years established these high pre-vaccination titers. Hence we were not able to conclude on age-specific responses to H1N1 or use this data for vaccine response stratification. Contrarily, a slight change in the H3N2 subclades took place in the 2019–2020 season29, potentially limiting pre-vaccination immunity. To fully understand the effect of pre-vaccination immunity on QIV responsiveness, also influenza-specific cross-reactive T-cells should be carefully examined30. This investigation is of special relevance due to the currently acknowledged large impact of aging on the T-cell compartment31.
The lower antibody concentrations following the primary PCV13 and mRNA-1273 vaccinations as well as the reduced breadth of response following PCV13 vaccination in older adults fits with the general concept of reduced potential to respond to de novo antigens at older age32 and potentially relates to a general decline of naïve immune cells with advancing age33,34,35. In line with this, lower responses following primary vaccinations, such as SARS-CoV-25,36, pneumococci37,38, yellow fever39, and Japanese encephalitis40 vaccinations in older adults were previously observed by others. Nevertheless, the comparison of primary vaccine responses between younger and older adults is often complicated by a difference in pre-vaccination immunity induced by historical natural exposures. Although PCV13 vaccination was a primary challenge in this cohort, previous natural exposure is evidenced by a large variation in serotype-specific pre-vaccination IgG concentrations between individuals and expected due to the high natural circulation of pneumococci41. These previous exposures likely influenced pre-vaccination humoral and cellular immunity and the response to the different serotypes in the PCV13 vaccine. For example, the absence of an age effect for serotype 14 is likely explained by higher pre-vaccination immunity caused by natural exposure to this serotype in the older age groups. Moreover, differences in immunogenicity between serotypes, as generally acknowledged for serotype 342, likely influenced the percentage of responders and height of the antibody concentrations. Interestingly, despite high and stable serotype 19A specific responses between age groups, this serotype was one of the major serotypes causing invasive pneumococcal disease (IPD) in the past years43. Hence, these serotype-specific differences warrant further investigation.
The observation of a slower induction of PCV13-specific antibodies in older adults either supports a difference in pre-vaccination immunity between the age groups or indicates a delay in immune responses at older age, as previous also observed following yellow fever vaccination39. To unravel the mechanisms underlying these delayed responses in older adults, as well as the age groups specific correlation between serotype-specific IgG responses, investigation of cellular pre-vaccination immunity is warranted. Specifically, in-depth investigation of pneumococcal-specific cellular immunity, but also immunity against CRM197, the conjugate, in the different age groups will help to unravel the mechanisms underlying the deviating immune responses observed to PCV13 vaccination in older adults.
The SARS-CoV-2 vaccination campaign provided a unique opportunity to investigate an additional primary vaccine response in relation to age. Despite adequate vaccine responses in all age groups, a strong trend towards a lower peak antibody response was observed in the older adults, which is in agreement with previous findings5. Noteworthy, the exclusion of the oldest individuals, due to the administration of a primary series of BNT162b2 vaccination to these individuals in the general vaccination campaign of the Netherlands preceding our vaccination initiatives, likely impacted the statistical power of this comparison.
Besides lower peak antibody responses to PCV13 and mRNA-1273 vaccination, our results also indicate a shorter persistence of humoral responses in older adults following all three vaccinations. This finding might be explained by an age-related decline in the survival of long-lived plasma cells due to decreased survival niches for long-lived plasma cells in the aging bone marrow44. Secondly, this observation is indicative of a diminished memory B cells response, potentially due to the involvement of Age-Associated B (AAB) cells45, as was also previously noted following vaccination in older age groups46,47. Therefore, more frequent booster vaccinations might be desirable at older age to maintain long-term protection.
The enhanced variation in antibody titers induced by the different vaccinations in the older age groups emphasizes the currently acknowledged deviating pace of immunosenescence between aging adults17, and hence supports the use of risk profiles, instead of chronological age, in the design of future vaccination strategies for the aging population. Early signs of aging immunity are already visible in the middle-aged adults group, as evidenced by the slightly lower antibody responses and the reduced breadth of the response to PCV13 vaccination as compared to the young adults. Similarly, lower vaccine responses towards a primary meningococcal vaccine were previously found in middle-aged adults as compared to adolescence48. Nevertheless, only a few middle-aged adults were found amongst the lowest dual and triple vaccine responders and hence middle-aged adults are an interesting target for future vaccine strategies, in order to strengthen memory immunity in the general population before reaching older age49,50.
Importantly, the unique setup of the presented clinical trial allowed investigation of vaccine responsiveness transcending over multiple vaccines and aimed to identify risk groups for low responsiveness. This novel head-to-head comparison of humoral immunity to multiple vaccines within the same individual revealed variable vaccine response patterns between individuals of similar age.
The contradictory classification of some individuals as high responders following QIV vaccination and at the same time low responders following PCV13 and mRNA-1273 vaccination, suggests that this vaccine-specific responsiveness is partly explained by the nature of the induced immune response; either a booster or a primary response, in which pre-vaccination immunity is crucial. Due to limited statistical power, we were not able to pinpoint exact associations between demographic characteristics and the multiple vaccine response patterns we observed46.
Yet, 8.9% (low triple vaccine response score) to 16.8% (cluster 4, low responders) of individuals, primarily of the male sex, was classified as overall low responder to multiple vaccines. Previously, sex has been described as an important parameter in vaccine responsiveness51 as well as immune ageing14,15,52. Moreover, a faster pace of immunological aging has been suggested in older males53,54. Remarkably, these older male adults also possessed lower pre-vaccination H3N2 titers, a phenomenon more often observed in older male adults55. Hence, the male sex and low pre-vaccination immunity might indicate combined risk factors for low vaccine responsiveness at older age.
Contrary to previous research that observed a correlation between the Frailty Index and humoral responses to SARS-CoV-2 vaccination in older adults56, no association between the Frailty Index and vaccine responsiveness was observed. The size of our cohort and the relatively healthy status of the participants, in which nursing home residents were excluded, likely affect our analysis. Yet, it is of interest to investigate whether parameters more closely resembling the immune status could predict vaccine responsiveness.
Besides the unique setup, this study has several limitations. Firstly, the use of different humoral response units complicated the analysis of multiple vaccine responsiveness. However, employing the vaccine scoring method allowed the investigation of humoral vaccine response transcending over multiple vaccines. Secondly, as a result of the COVID-19 lockdowns, blood sampling timepoints have been postponed, increasing the sampling window of the long-term response following QIV vaccination. Nevertheless, long-term H3N2 HI titers could still be compared between the age groups, due to an equal sampling distribution in the age groups. Moreover, due to the high proportion of healthcare workers amongst the young and middle-aged adults, this cohort might not fully represent the general population. Finally, the current analysis is performed on antibody concentrations only. Despite previously observed high correlations between antibody concentrations and vaccine efficacy57,58, it is of interest to also investigate antibody functionality and cellular immunity in relation to aging.
Taken together, the outcomes indicate a potential large impact of pre-vaccination immunity on vaccine responsiveness at older age. Moreover, the presented study accommodates the identification of risk groups for low vaccine responsiveness. In order to move towards more targeted vaccination strategies for the rapidly aging population, future research should next aim to untangle the mechanisms underlying these low responses.
Methods
Study design and participants
We here report on the longitudinal intervention studies VITAL and VITAL-corona21. Samples of these primary endpoints were collected between 2019 and 2021. Within this cohort, participants divided over 3 age groups were recruited: young adults (25–49 y), middle-aged adults (50–64 y), and older adults (≥65 y). The young and middle-aged adults were recruited among workers of public health institutions of the University Science Park and University Medical Center Utrecht, The Netherlands. Older adults were recruited from a previous cohort59,60. All participants needed to be capacitated and vaccinated with the seasonal influenza vaccination in season 2018–2019 to be considered eligible for participation. At the start of the intervention cohort (autumn 2019), potential participants were excluded based on the following criteria: received a previous pneumococcal vaccination, known or suspected allergy to any of the vaccine components, received a high systemic (>20 mg) daily dose of corticosteroids within 2 weeks before inclusion, use of high (>30 mg) dose of corticosteroids in the recent past, recipient of an organ or bone marrow transplant, have a (functional) asplenia, received chemotherapy in the past 3 years, received blood products or immunoglobulins within 3 months before inclusion, known or suspected coagulation disorder that would contraindicate against intramuscular injection and frequent blood sampling, known or suspected immunodeficiency or use of immunosuppressive therapy, known anemia, or known infection with immunodeficiency virus (HIV) and/or hepatitis B and/or C virus. Participants were additionally excluded for the mRNA-1273 vaccination when having received treatment with COVID-19 monoclonal antibodies within 3 months before vaccination. Moreover, participants were temporarily excluded from the study when they: received any vaccine within 1 month of a vaccination visit or within 2 weeks of blood collection. Study visits were postponed when participants were experiencing an elevated body temperature >38 °C within 72 h before a vaccination visit or 48 h before a blood collection visit as well as in case of a positive COVID-19 test (visit postponed for at least 4 weeks). Finally, participants were withdrawn from the study when: receiving a systemic high (>20 mg) dose of corticosteroids, starting chemotherapy treatment, receiving blood products or immunoglobulins, being pregnant at the moment of pneumococcal or mRNA-1273 vaccination, or perceiving sudden anemia. Participants received a small financial compensation for their time investment.
Ethical approval was obtained through the Medical Research Ethics Committee Utrecht (NL69701.041.19, EudraCT: 2019-000836-24). All participants provided written informed consent and all procedures were performed with Good Clinical Practice and in accordance with the principles of the Declaration of Helsinki.
Vaccinations and blood sampling
A schematic outline of the study design is depicted in Fig. 1A. At the start of the study, a blood sample was collected from all participants (pre-QIV; start summer 2019). Subsequently, all participants were vaccinated with Influvac Tetra (2019–2020) (autumn 2019); the seasonal quadrivalent inactivated subunit influenza vaccine (QIV)(2019–2020), containing neuraminidase and hemagglutinin from the following viral strains: A/Brisbane/02/2018, IVR-190(H1N1); A/Kansas/14/2017, NYMC X-327 (H3N2); B/Maryland/15/2016, NYMC BX-69A (B/Victoria/2/87 lineage); and B/Phuket/3073, wildtype (B/Yamagata/16/88 lineage) (Abbott Biologicals B.V. The Netherlands).
Secondly, during the summer/autumn of 2020, all participants were vaccinated with Prevenar 13, the 13-valent pneumococcal polysaccharide conjugate vaccine containing polysaccharides from the pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F conjugated to the CRM197 carrier protein (Pfizer Europe, Belgium).
Finally, during spring 2021, participants received a primary vaccination series with one-month interval with Spikevax, the SARS-COV2 mRNA-1273 vaccine (Moderna Biotech, Spain), unless they already had been vaccinated through the national vaccination program with a BNT162b2 vaccine.
Following each vaccination blood samples were collected 28 days (±3 days) and 6 months (range 5–8 months) post-vaccination. Here the 6-month timepoints also serve as pre-vaccination sample before either PCV13 or mRNA-1273 vaccination. In addition, a blood sample was collected 7 days (±1 day) following PCV13 vaccination and at the moment of the second mRNA-1273 vaccination. Of note, in spring 2020, the SARS-COV2 pandemic hit, resulting in a temporary shutdown of the cohort and restart 4 months later. Therefore, the 6 months (window 5–8 months) post-QIV vaccination sampling was extended to a window of 12 months.
Blood samples were collected by venipuncture using blood collection tubes containing clot activator and gel separator (Vacuette tubes, Article number 455071, Greiner Bio-one, Austria). Serum was collected and aliquoted within 8 h after sampling and stored at −80 °C until further use.
Serological analysis
The humoral response towards the influenza A strains A/Brisbane/02/2018, IVR-190(H1N1) and A/Kansas/14/2017, NYMC X-327 (H3N2) strain at the pre- and post-vaccination timepoints were used to evaluate the response towards the QIV vaccination. The H1N1 and H3N2-specific antibody responses were respectively measured at Vismederi (Siena, Italy) and Viroclinics (Rotterdam, the Netherlands) using the Hemagglutination Inhibition (HI) assay, the most commonly used assays for measuring influenza-specific antibody titers, according to the standard methods of the World Health Organization (WHO) as explained in refs. 61,62,63. In brief, a dilution series of serum samples was incubated with Hemagglutinin Units (HAU) influenza virus and thereafter incubated with turkey erythrocytes. Subsequently, the agglutination of red blood cells was scored and the antibody titer preventing agglutination calculated. An HI titer >40 was considered protective. A response to the QIV vaccine was defined as an HI titer >40 at 28 days post-vaccination and a fold increase of >4 compared to the pre-QIV timepoint.
The pneumococcal serotype-specific IgG concentrations for the 13 serotypes present in the PCV13 vaccine were measured using the fluorescent-bead-based-multiplex immunoassay (MIA) as previously described in refs. 64,65 with minor modifications of using the protein-free buffer SurModics® Assay Diluent (SM01, Surmodics IVD Inc, USA) with 10% FCS in the assay. The WHO international standard 007sp was used as a standard. For each sample, median fluorescent intensity was converted to IgG concentration (μg/ml) by interpolation from a 5-parameter logistic standard curve. Results were obtained using a Bio-plex 200 system with Bio-plex software (version 6.2, Bio-Rad, UK).
The Spike S1-specific IgG concentrations induced by the mRNA-1273 vaccine were measured using bead-based assay as previously described66. Here the S1-specific concentrations were calibrated against the SARS-CoV2 specific international standard (20/136 NIBSC standard, National Institute for Biological Standards and Control, UK) and expressed as binding antibody units/mL (BAU/mL) and a concentration of 10.1 BAU/mL was used a cut-off for seropositivity.
Vaccine scoring method
In order to define an individual’s vaccine responsiveness towards multiple vaccines, an individual vaccine response score was defined per vaccine.
In order to do so, the 28 days post-vaccination antibody titers per vaccine antigen (QIV: the H3N2 titer, PCV13: the IgG concentrations against the 13 pneumococcal serotypes, and mRNA-1273: the IgG concentrations against the Spike S1 protein) were divided into quartiles. Subsequently, a score of 1 was given to an individual with an antibody titer in the lowest quartile, whereas a score of 4 was given to an individual with an antibody titer in the highest quartile (and 2 and 3 to the middle quartiles).
Since for the PCV13 vaccine, antibody responses were measured for 13 different strains separately, we first defined the response score per serotype. Thereafter we used a majority votes approach (most frequent score among the 13 serotypes), to define the most frequent response score among the 13 serotypes and used this most frequent score as the overall vaccine response score. To break ties for a few cases, where individuals had similar frequency of scores, we randomized the score selection using the mclust package (v5.4.6) majority Vote function in R (v4.3.2).
An individual’s dual or triple vaccine response score was defined as the average between the scores to the QIV and PCV13 (dual) or QIV, PCV13, and mRNA-1273 (triple), respectively. Of note, since the triple vaccine response score was investigated in a smaller group of individuals, the quartile division slightly differed between the dual and triple vaccine analyses.
Frailty status determinants
The Frailty Index, EQ-5D-3L questionnaire score, and number of prescription medications were used to assess the frailty status of the participants and were compared between the dual and triple vaccine response groups. The Frailty Index and EQ-5D-3L scores in the VITAL cohort have been described previously67. In brief, the Frailty Index is based on 31 deficits and the scores ranged from 0 (least frail) to 0.53 (most frail) in this cohort. The EQ-5D-3L index and number of prescription medications are based on self-reported questionnaires. Answers to the EQ-5D-3L questionnaire were converted using the Dutch population norms, resulting in scores ranging from 1 (least frail) to -.03 (most frail) in this cohort. The number of medications included all medications prescribed by a physician.
Data analysis
The distribution of data was tested before downstream analysis. Pre- and 28 days post- vaccination antibody levels were compared between the age groups using the Kruskall–Wallis test (kruskal_test function) and corrected for multiple comparisons with Bonferroni correction. The variation in antibody concentrations between age groups was compared with the LeveneTest function. When comparing paired samples from individuals at two different timepoints, the Wilcoxon signed rank test (wilcox_test function) was applied. The above-mentioned analyses, as well as the calculation of the mean (QIV) and geometric mean (PCV13 and mRNA-1273) titers and 95% Confidence intervals (CI) per age group, were performed in R studio (v4.2.3) using the Tidyverse package (v2.0.0) and using 999 bootstrap replicates.
Humoral responses were compared between males and females within every age group with the Mann–Whitney U test and the proportion of responders between age groups and sexes with the Chi-Squared test. These analyses were performed in GraphPad Prism (v8.0.1).
Association between log-transformed longitudinal vaccine response and the interaction of timepoints (QIV and mRNA-1273: pre-, 28 days and 6 months, PCV13: pre-, 7 days, 28 days and 6 months) and age groups (YA, MA, and OA) was assessed by means of linear mixed models. The random effect structure comprised a random intercept for time. Model checking was done by means of visual assessment of residuals plots. The R (v4.3.2) package nlme (v3.1-164) was used for model fitting and the package effects (v4.2.2) was used to derive the predicted mean responses on the original scale with their 95% confidence intervals.
Correlations between the 28 days post-vaccination antibody titers were calculated using Spearman correlation (GraphPad Prism (v8.0.1)).
Unsupervised agglomerative hierarchical clustering was performed using Ward’s method and Manhattan distance to allow for the clustering of ordinal stepwise vaccine response scores. The optimal number of clusters was determined with the elbow method. The clustering analyses were exclusively performed in R (v4.3.2). First, the Manhattan distances were calculated using the dist function from the base stats package (v4.3.2). These distances were then supplied to the agnes function from the cluster package (v2.1.4) for performing the agglomerative hierarchical clustering using Ward’s method. The optimal number of clusters was inferred through the fviz_nbclust function from the factoextra package (v1.0.7), here the maximum number of clusters (k.max) was set to 20. The heatmap visualization of the optimal number of clusters was performed using the pheatmap function of the pheatmap package (v1.0.12).
Comparison of demographic characteristics between the dual and triple vaccine response groups and clusters were performed using the Kruskall–Wallis test and corrected for multiple comparisons with Bonferroni correction in GraphPad prism (v8.0.1). Sex was compared between the vaccine response groups and clusters with the Chi-Squared test. In all analyses, a p-value < 0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets containing participant-specific data used in the current studies are available under restricted access since (1) the study is still ongoing and (2) to comply with EU legislation on the General Data Protection Regulation (GDPR) and participant privacy and ethical rights. All data used in the figures and tables is provided in the Source data file. However, data rows are displayed in random order and some datapoints have been redacted due to GDPR considerations. Access to the redacted data can be obtained via the corresponding author in the form of pseudonymized data as long as data transfer is in agreement with the clinical protocol and GDPR and takes into account participants’ privacy and ethical rights. Data sharing will be regulated in a data sharing agreement in the timeframe of 2 months after receipt of the request. Source data are provided with this paper.
References
Pollard, A. J. & Bijker, E. M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 21, 83–100 (2021).
Chang, A. Y., Skirbekk, V. F., Tyrovolas, S., Kassebaum, N. J. & Dieleman, J. L. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health 4, e159–e167 (2019).
Rappuoli, R., Mandl, C. W., Black, S. & De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 11, 865–872 (2015).
Niederman, M. S. et al. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: a review of available evidence. Expert Rev. Vaccines 20, 243–256 (2021).
van den Hoogen, L. L. et al. Reduced antibody acquisition with increasing age following vaccination with BNT162b2: results from two longitudinal cohort studies in The Netherlands. Vaccines 10, 1480 (2022).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Kimball, J., Zhu, Y., Wyatt, D., Trabue, C. H. & Talbot, H. K. Influenza vaccine failure associated with age and immunosuppression. J. Infect. Dis. 224, 288–293 (2020).
Nanishi, E., Levy, O. & Ozonoff, A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: a rapid review. Hum. Vaccines Immunother. 18, 2045857 (2022).
McElhaney, J. E. et al. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing 17, 1–10 (2020).
Cevirgel, A. et al. Identification of aging-associated immunotypes and immune stability as indicators of post-vaccination immune activation. Aging Cell 21, 1–14 (2022).
Pawelec, G. & Solana, R. Immunosenescence. Immunol. Today 18, 514–516 (1997).
Pawelec, G. et al. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 192, 111357 (2020).
Fulop, T. et al. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front. Immunol. 8, 1960 (2018).
Caruso, C., Accardi, G., Virruso, C. & Candore, G. Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun. Ageing 10, 20 (2013).
Bupp, M. R. G., Potluri, T., Fink, A. L. & Klein, S. L. The confluence of sex hormones and aging on immunity. Front. Immunol. 9, 1269 (2018).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Goudsmit, J. et al. Immune age and biological age as determinants of vaccine responsiveness among elderly populations: the Human Immunomics Initiative research program. Eur. J. Epidemiol. 36, 753–762 (2021).
Rubino, G. et al. Sicilian centenarian offspring are more resistant to immune ageing. Aging Clin. Exp. Res. 31, 125–133 (2019).
Hoogendijk, E. O. et al. Frailty: implications for clinical practice and public health. Lancet 394, 1365–1375 (2019).
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
Van Baarle, D. et al. Preventing infectious diseases for healthy ageing: The VITAL public-private partnership project. Vaccine 38, 5896–5904 (2020).
Haggenburg, S. et al. Immunogenicity of the 13-valent pneumococcal conjugated vaccine followed by the 23-valent polysaccharide vaccine in chronic lymphocytic leukemia. Vaccines 11, 1201 (2023).
Daly, T. M. & Hill, H. R. Use and clinical interpretation of pneumococcal antibody measurements in the evaluation of humoral immune function. Clin. Vaccin. Immunol. 22, 148–152 (2015).
Orange, J. S. et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 130, S1–S24 (2012).
Andrew, M. K. et al. Influenza vaccination in older adults: recent innovations and practical applications. Drugs Aging 36, 29–37 (2019).
Lang, P. O. et al. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin. Inter. Aging 7, 55–64 (2012).
Dugan, H. L., Henry, C. & Wilson, P. C. Aging and influenza vaccine-induced immunity. Cell. Immunol. 348, 103998 (2020).
Ganczak, M. et al. Quadrivalent influenza vaccine-induced antibody response and influencing determinants in patients ≥ 55 years of age in the 2018/2019 season. Int. J. Environ. Res. Public Health 16, 4489 (2019).
World Health Organization. Addendum to the Recommended Composition of Influenza Virus Vaccines for Use in the 2019-2020 Northern Hemisphere Season (2019).
Gaevert, J. A., Duque, D. L., Lythe, G., Molina-París, C. & Thomas, P. G. Quantifying t cell cross-reactivity: Influenza and coronaviruses. Viruses 13, 1786 (2021).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Lang, P. O. & Aspinall, R. Immunosenescence and herd immunity: with an ever-increasing aging population do we need to rethink vaccine schedules? Expert Rev. Vaccines 11, 167–176 (2012).
Van Den Broek, T., Borghans, J. A. M., & Van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 18, 363–373 (2018).
den Braber, I. et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 36, 288–297 (2012).
Egorov, E. S. et al. The changing landscape of naive T cell receptor repertoire with human aging. Front. Immunol. 9, 1–12 (2018).
Demaret, J. et al. Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front. Immunol. 12, 778679 (2021).
Van Werkhoven, C. H., Huijts, S. M., Bolkenbaas, M., Grobbee, D. E. & Bonten, M. J. M. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin. Infect. Dis. 61, 1835–1838 (2015).
Park, S. & Nahm, M. H. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect. Immun. 79, 314–320 (2011).
Roukens, A. H. et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS ONE 6, e27753 (2011).
Wagner, A. et al. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci. Rep. 8, 9825 (2018).
Vissers, M. et al. Increased carriage of non-vaccine serotypes with low invasive disease potential four years after switching to the 10-valent pneumococcal conjugate vaccine in The Netherlands. PLoS ONE 13, e0194823 (2018).
Khatun, F., Stephenson, R. J. & Toth, I. An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chem. A Eur. J. 23, 4233–4254 (2017).
Dutch National Institute of Public Health and the Environment. The National Immunisation Programme in the Netherlands. Surveillance and Developments in 2022–2023 (2023).
Pritz, T. et al. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 45, 738–746 (2015).
Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315–340 (2020).
Burton, A. R. et al. The memory B cell response to influenza vaccination is impaired in older persons. Cell Rep. 41, 111613 (2022).
Siegrist, C. A. & Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9, 185–194 (2009).
van der Heiden, M. et al. Lower antibody functionality in middle-aged adults compared to adolescents after primary meningococcal vaccination: role of IgM. Exp. Gerontol. 105, 101–108 (2018).
van der Heiden, M. et al. Novel intervention in the aging population: a primary meningococcal vaccine inducing protective IgM responses in middle-aged adults. Front. Immunol. 8, 817 (2017).
van der Heiden, M. et al. An explorative biomarker study for vaccine responsiveness after a primary meningococcal vaccination in middle-aged adults. Front. Immunol. 8, 1962 (2018).
Klein, S. L., Jedlicka, A. & Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10, 338–349 (2010).
Márquez, E. J. et al. Sexual-dimorphism in human immune system aging. Nat. Commun. 11, 751 (2020).
Hirokawa, K. et al. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 10, 19 (2013).
Van Der Heiden, M. et al. Differential effects of Cytomegalovirus carriage on the immune phenotype of middle-aged males and females. Sci. Rep. 6, 26892 (2016).
Shapiro, J. R. et al. Sex-specific effects of aging on humoral immune responses to repeated influenza vaccination in older adults. NPJ Vaccines 6, 147 (2021).
Kuijpers, Y. et al. Potential determinants of antibody responses after vaccination against SARS-CoV-2 in older persons: the Doetinchem Cohort Study. Immun. Ageing 20, 57 (2023).
Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39, 4423–4428 (2021).
Wei, J. et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 28, 1072–1082 (2022).
Kaaijk, P. et al. Contribution of influenza viruses, other respiratory viruses and viral co-infections to influenza-like illness in older adults. Viruses 14, 797 (2022).
Van Beek, J. et al. Influenza-like illness incidence is not reduced by influenza vaccination in a cohort of older adults, despite effectively reducing laboratory-confirmed influenza virus infections. J. Infect. Dis. 216, 415–424 (2017).
Luytjes, W. et al. HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non-homologous strains. Vaccine 30, 5262–5269 (2012).
Rosendahl Huber, S. K. et al. Immunogenicity of influenza vaccines: evidence for differential effect of secondary vaccination on humoral and cellular immunity. Front. Immunol. 9, 3103 (2018).
ECDC. Immunity following influenza disease and administration of influenza vaccines. https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/immunity (2018).
Elberse, K. E. M. et al. Seroprevalence of IgG antibodies against 13 vaccine Streptococcus pneumoniae serotypes in the Netherlands. Vaccine 29, 1029–1035 (2011).
Elberse, K. E. M., Tcherniaeva, I., Berbers, G. A. M. & Schouls, L. M. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccin. Immunol. 17, 674–682 (2010).
Den Hartog, G. et al. SARS-CoV-2-specific antibody detection for seroepidemiology: a multiplex analysis approach accounting for accurate seroprevalence. J. Infect. Dis. 222, 1452–1461 (2020).
van Sleen, Y. et al. Frailty is related to serum inflammageing markers: results from the VITAL study. Immun. Ageing 20, 68 (2023).
Acknowledgements
We would like to thank all collaborators; Inge Pronk, Linde Woudstra, Jacqueline Zonneveld, Sandra Hoogkamer, Marjolein Izeboud, Olga de Bruin, Helma Lith, Joyce Geeber, Ilse Akkerman, Megan Barnes, Lysanne Bakker, Shirley Man, Silvia Cohen, Ruben Wiegmans, Nazela Mir Leibady, Ronith van de Wiele, Rydianne Carvalho De Moura, Emma van Wijlen, Petra Molenaar, Madelène Paets, Martien Poelen, Martijn Vos, Jeroen Hoeboer, Marion Hendriks, Noortje Smits, Jolanda Kool, Maarten Emmelot, Ronald Jacobi, Stefanie Lenz, Floor Peters, Gaby Smits, Marjan Kuijer, Manas Joshi and Alina Nicolaie. The VITAL project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No. 806776 and the Dutch Ministry of Health, Welfare and Sport (D.v.B.). The JU receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA‐members. The VITAL- Corona study was funded by the Dutch Ministry of Health, Welfare, and Sport (NR).
Author information
Authors and Affiliations
Contributions
L.B., M.v.H., N.R., W.B., J.v.B., and D.v.B. designed the study, wrote the medical ethical application, and performed the clinical trial. L.B., I.T., R.v.B., T.O., A.B., and J.v.B. were involved in data acquisition. E.B. was responsible for the data management. M.v.d.H., S.S., E.B., A.C., Y.v.S., and J.v.B. analyzed the data. M.v.d.H., J.v.B., and D.v.B. wrote the manuscript. All authors critically revised the manuscript before publication.
Corresponding author
Ethics declarations
Competing interests
T.O. and W.B. are employees of the GSK group of companies. W.B. holds shares in the GSK group of companies. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Rodolphe Thiébaut, Sophie Valkenburg, and Nicholas Wohlgemuth for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
van der Heiden, M., Shetty, S., Bijvank, E. et al. Multiple vaccine comparison in the same adults reveals vaccine-specific and age-related humoral response patterns: an open phase IV trial. Nat Commun 15, 6603 (2024). https://doi.org/10.1038/s41467-024-50760-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50760-9
- Springer Nature Limited