Abstract
The interactions between adsorbed gas molecules within porous metal-organic frameworks are crucial to gas selectivity but remain poorly explored. Here, we report the modulation of packing geometries of CO2 and C2H2 clusters within the ultramicroporous CUK-1 material as a function of temperature. In-situ synchrotron X-ray diffraction reveals a unique temperature-dependent reversal of CO2 and C2H2 adsorption affinities on CUK-1, which is validated by gas sorption and dynamic breakthrough experiments, affording high-purity C2H2 (99.95%) from the equimolar mixture of C2H2/CO2 via a one-step purification process. At low temperatures (<253 K), CUK-1 preferentially adsorbs CO2 with both high selectivity (>10) and capacity (170 cm3 g−1) owing to the formation of CO2 tetramers that simultaneously maximize the guest-guest and host-guest interactions. At room temperature, conventionally selective adsorption of C2H2 is observed. The selectivity reversal, structural robustness, and facile regeneration of CUK-1 suggest its potential for producing high-purity C2H2 by temperature-swing sorption.
Similar content being viewed by others
Introduction
Host-guest chemistry is fundamental to the selectivity of many molecular recognition systems1,2,3,4,5. The optimization of cooperative interactions, such as electrostatic interactions and hydrogen bonding, plays a crucial role in the design of efficient molecular recognition systems, particularly in porous materials. These cooperative interactions are essential for achieving high performance in gas adsorption, sensing, and catalysis applications1,3,6,7,8,9,10,11,12,13,14,15,16,17. On the other hand, guest-guest interactions or the formation of guest clusters also play an important role in molecular recognition. However, the direct observation and control of guest-guest interactions within confined nanovoids of porous materials is highly challenging and remains poorly explored18,19,20. Screening new host-guest and guest-guest interactions can promote the design of new functional porous materials1,21.
Ultramicroporous metal-organic frameworks (MOFs), featuring highly inerratic porosity, tunable pore chemistry, and designable structures, provide a unique platform to explore host-guest interactions11,22,23,24,25,26,27. In particular, the modular nature and reticular structure endow ultramicroporous MOFs with the possibility to precisely control the host-guest and guest-guest interactions within the pores28,29. Great advances in host-guest chemistry have been achieved in ultramicroporous MOFs with tailor-made properties for gas adsorption and separation, owing to the confinement effect from the strong host-guest interactions30,31,32. Currently, the major interest in gas adsorption and separation using ultramicroporous MOFs is focused on enhancing recognition selectivity by tuning the host-guest interactions19,29,33,34,35,36. This is pronounced for selective adsorption of acetylene (C2H2) from carbon dioxide (CO2), as C2H2 is one of the most important industrial precursors, and the CO2 contaminant would be coproduced during the production of C2H2 via partial combustion of natural gas34,35,36. However, the understanding of the impact of guest-guest interactions or guest clusters on selectivity remains lacking due to the difficulties in the direct observation of such dynamic and weak interactions.
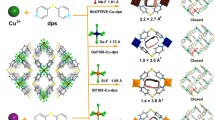
Herein, we report the modulation of geometries of guest-clusters as a function of temperature (Fig. 1) for the normal and inverse selectively and separation of CO2 and C2H2 within the robust ultramicroporous M-CUK-1 (M = Co, Ni, and Mg) materials. The guest-guest interactions and binding domains within CUK-1 with different metal nodes have been observed by in-situ synchrotron X-ray diffractions and molecular simulations. The efficient packing of well-organized CO2 clusters with T-shaped dimers gives rise to notably higher crystallographic occupancy and capacity of CO2 (106 vs. 86 cm3 g−1 of C2H2 in Co-CUK-1 at 298 K), while the stronger host-guest interactions between C2H2 and CUK-1 at room temperature lead CUK-1 to preferentially adsorb C2H2 over CO2 (Fig. 1). Notably, a much larger increment of CO2 capacities at low temperatures was observed compared with those of C2H2, which is resulted from the highly efficient packing of CO2 clusters with tetramers and the significantly stronger host-guest interactions between CO2 and CUK-1. This finally leads to much higher CO2 capacities (170 vs. 119 cm3 g−1 of C2H2 at 233 K) and clear sorption inversion of CO2 over C2H2. Such an inverse CO2/C2H2 adsorption behavior is more desirable for industrial production of C2H2 via a one-step CO2 adsorption process but is rarely investigated18,29,37,38,39,40. The temperature-dependent reversal of sorption behavior for CO2 and C2H2 is demonstrated by gas sorption isotherms and dynamic breakthrough experiments at various temperatures. High-purity C2H2 (99.995%) can be directly obtained in a one-step process, and the low energy input for the regeneration suggests that CUK-1 is a promising adsorbent for C2H2 production via the temperature-swing adsorption (TSA) process.
At a high temperature (T1, purple), the strong host-guest and guest-guest interactions result in preferential adsorption of C2H2, but the efficient packing of molecular chains formed by CO2 molecules through strong guest-guest interactions leads to the higher uptake at the high-pressure range (>Pcross). After decreasing the temperature to T2 (blue), the CO2 clusters with T-shaped dimers exhibit higher occupancy of the pore channels than that of C2H2 clusters packed together via π⋯π interactions, coupled with the strong host-guest interactions, leading to the inverse CO2 preferential sorption.
Results
Materials and characterization
M-CUK-1 (M = Co, Ni, and Mg) were hydrothermally synthesized by reacting 2,4-pyridinedicarboxylic acid (2,4-H2pdc) and M2+-containing salts (M = Co, Ni, and Mg) with KOH in water at 210 °C for 24 h41,42,43. The CUK-1 materials are isostructural. The edge- and vertex-sharing M3(µ3-OH)2 chains serve as undulating pillars connecting the 2,4-pdc ligands in an orthogonal fashion, forming a ‘wine-rack’ topology with one-dimensional diamond-shaped and corrugated channels (Supplementary Fig. 1)41,42,43. All three CUK-1 materials show excellent chemical and structural stability, and are entirely stable upon air exposure for two years (Supplementary Figs. 2–5). Desolvated CUK-1 exhibits an ultramicroporous structure, as evidenced by the negligible N2 uptakes and typical type-I CO2 isotherms at 77 K and 196 K, respectively (Supplementary Figs. 6–9). The calculated Brunauer–Emmett–Teller (BET) surface areas are 500~600 m2 g−1 based on the CO2 isotherms. Upon desolvation, the exposed μ3-OH groups reside orderly in the channels (8.1 × 10.6 Å2, Supplementary Fig. 1), acting as potential binding sites to guest molecules through electrostatic interactions41,42,43. This is highly desirable for the adsorption and separation of hydrocarbons.
Analysis of gas adsorption isotherms and selectivity
Adsorption isotherms of CO2 and C2H2 on desolvated M-CUK-1 (M = Co, Ni, and Mg) at 298 K indicate the preferential adsorption of C2H2 at low pressure but higher saturation capacity of CO2 upon increasing the pressure (Fig. 2a–c). This behavior results in the intersection of the two isotherms at moderate pressure. Another ultramicroporous compound, SIFSIX-3-Ni, exhibits similar isotherm crossing but with a stronger affinity to CO2 at low pressures29. After the intersection of CO2 and C2H2 isotherms at 0.42 bar on Co-CUK-1, the CO2 isotherm is above that of C2H2, and CO2 uptake at 1 bar can reach 106 cm3 g−1, much higher than that of C2H2 (86 cm3 g−1, Fig. 2a). To the best of our knowledge, such an intersection between CO2 and C2H2 isotherms is rarely observed on porous materials29. Similarly, Ni- and Mg-CUK-1 show stronger sorption affinities to C2H2 in the low-pressure range, and the CO2 and C2H2 isotherms also intersect but with relatively higher intersecting pressures of 0.6 and 0.95 bar on Ni- and Mg-CUK-1, respectively (Fig. 2b, c).
The CO2 and C2H2 adsorption isotherms on desolvated Co-CUK-1 (a), Ni-CUK-1 (b), and Mg-CUK-1 (c) at 298 (purple), 253 (blue), and 233 K (orange). d The comparison of CO2 and C2H2 uptakes at 0.5 bar on CUK-1 materials at different temperatures. e The inverse CO2/C2H2 (1/1) (top) and normal C2H2/CO2 (1/1) (bottom) selectivities at 233 and 298 K, respectively, on CUK-1 materials. f Comparison of the zero-coverage heat of adsorption of CUK-1 materials for CO2 with those of other materials for inverse CO2/C2H2 separation.
Considering that the inversed CO2/C2H2 selectivity is more desirable for industrial C2H2 production, the respective guest loadings were measured at progressively lower temperatures to decrease the intersecting pressures. Upon reducing the temperature, there are significant enhancements for CO2 uptakes but only a slight increase in C2H2 uptakes, finally leading to much higher CO2 uptakes, even at very low pressures (Fig. 2a–c and Supplementary Figs. 7–9). Specifically, the CO2 uptakes at 233 K are 170, 142, and 144 cm3 g−1 on Co-, Ni-, and Mg-CUK-1 (ca. 4.15, 3.43, and 3.50 CO2 molecules per cell, respectively), which notably exceed those of C2H2 (119, 97, and 89 cm3 g−1, respectively; ca. 2.89, 2.29, and 2.32 C2H2 molecules per cell on Co-, Ni-, and Mg-CUK-1, respectively). The densities of adsorbed CO2 molecules (based on the structural pore volume) in Co-, Ni-, and Mg-CUK-1 at 233 K were estimated to be 1.40, 1.27, and 1.25 g cm−3, respectively. Notably, these densities are higher than that of liquid CO2 (1.1 g cm−3) but lower than that of dry ice (1.55 to 1.7 g cm−3)44, indicating the highly efficient packing of CO2 in the pores. However, the densities of adsorbed C2H2 molecules were recorded as only 0.56, 0.50, and 0.45 g cm−3 in Co-, Ni-, and Mg-CUK-1, respectively, lower than that of liquid C2H2 (0.69 g cm−3)45. At 253 K and 233 K, there is a clear inversion in the adsorption selectivity from C2H2 to CO2 on the CUK-1 materials. The uptake gap between CO2 and C2H2 at 0.5 bar on Co-CUK-1 can reach 40 and 45 cm3 g−1 at 253 and 233 K (Fig. 2d), respectively.
State-of-the-art C2H2/CO2 separation is mainly realized by cryogenic distillation and solvent absorption with high energy penalty. The adsorptive separation using CO2-selective other than C2H2-selective materials is preferable in the industry for producing pure C2H2 via one-step sorption procedures. To see whether such a temperature-induced adsorption inversion behavior can be used for inverse CO2/C2H2 separation, we evaluated CUK-1 materials for separating the equimolar mixture of CO2/C2H2 by analyzing the single-component isotherms via ideal adsorbed solution theory (IAST). At 298 K, CUK-1 only shows a moderate C2H2/CO2 selectivity of ca. 2. However, at 233 K, CUK-1 exhibits the inversed CO2/C2H2 selectivity of 9.5, 8.4, and 12.1 for Co-, Ni-, and Mg-CUK-1, respectively (Fig. 2e). The inversed selectivities are comparable with those of the state-of-the-art materials for inversed CO2/C2H2 separation, such as [Mn(bdc)(bpe)] (9)46, Ce(IV)-MIL-140-4F (9.6)37, PCP-NH2-ipa (6.4)35, and SIFSIX-3-Ni (7.5)29, but lower than the benchmark material Cu-F-pymo (> 105)38. Furthermore, the isosteric heats of adsorption (ΔHads) of CO2 on Co- and Ni-CUK-1 were calculated to be 20.8 and 21.7 kJ mol−1, respectively (Supplementary Fig. 10), much lower than that of other materials (Fig. 2f), such as PCP-NH2-ipa (26.8 kJ mol−1)35, [Mn(bdc)(bpe)] (29.5 kJ mol−1)46, MUF-16 (32 kJ mol−1)39, and Tm2(OH-bdc) (45 kJ mol−1)40.
Guest configurations determined by in-situ synchrotron X-ray powder diffraction
In-situ synchrotron X-ray powder diffraction data on CO2- and C2H2-loaded CUK-1 materials were collected as a function of temperature (Supplementary Figs. 11–12). Full refinements of the data indicate two binding sites in the asymmetric unit: site I is close to the μ3-OH group, and site II locates near the pore surface (Figs. 3–4 and Supplementary Figs. 13–18). At 298 K, the total crystallographic occupancy of C2H2 molecules (2.05 per cell) in Co-CUK-1 is in excellent agreement with that obtained from the isotherm (2.07 C2H2 per cell). C2H2 molecules at site I locate almost perpendicular to μ3-OH groups, forming O-H⋯πC2H2 H-bonds (2.92 Å, dotted green lines), supplemented by additional interactions via C-HC2H2⋯Oligand H-bonding (dotted green lines, 2.67-2.71 Å, Fig. 3a and Supplementary Fig. 13a). C2H2 molecules at site II sit close to the aromatic rings on the pore surface and form weak interactions with the framework through multiple C-HC2H2⋯Oligand H-bonding (3.37–3.82 Å) and πC2H2⋯Hligand (3.44–3.67 Å) interactions. Moreover, at high loading, the neighboring C2H2 molecules synergistically interact with each other through multiple HC2H2⋯πC2H2 interactions (dotted pink lines, 2.33–2.95 Å), forming the tetramer-clusters of C2H2 (Fig. 3b).
In contrast, CO2 molecules show different geometries of packing (Fig. 3c and Supplementary Fig. 14a). CO2 molecules at site I exhibit an end-on interaction to μ3-OH group via hydrogen bonds (dotted lime lines, 2.42 Å of O-H⋯OCO2), but no interactions between CO2 and the ligand of CUK-1 were observed. CO2 molecules at site II interact with the pore surface via weak O⋯Hligand interactions (3.88–3.91 Å, Supplementary Fig. 14a). Thus, adsorbed CO2 molecules at both sites show much weaker interactions compared with C2H2, entirely consistent with the adsorption results at room temperature. However, at high loading, two one-dimensional chains of CO2 (dotted azure lines) running along the channel were formed via strong guest-guest interactions (2.93 and 2.74 Å, Fig. 3d). These chains are stabilized by multiple weak intermolecular dipole interactions between monomer-to-dimer and dimer-to-dimer of CO2. Furthermore, two chains interact with each other via multiple synergistic host-host interactions (dotted green lines, 3.27–3.28 Å). Notably, the neighboring CO2 molecules exhibit a head-to-center (C = O⋯C) geometry (Supplementary Fig. 14a), thus leading to the efficient packing of CO2 in the pore channels. Similar binding sites of CO2 in Ni-CUK-1 were also observed (Supplementary Fig. 18). Compared with C2H2 clusters, the efficient packing of CO2 molecules near the center of pore channels via strong guest-guest interactions but with less host-guest interactions is the main reason for the high adsorption of CO2 in CUK-1 at high pressures.
At 233 K, remarkable changes in the packing geometry of CO2 and C2H2 were observed (Fig. 4), and the crystallographic occupancy of C2H2 molecules increased to 2.77 per cell. Meanwhile, the CO2 occupancy increased to 3.47 per cell (vs. 2.07 at 298 K), indicating the high capacity of Co-CUK-1 for CO2 at 233 K compared with that for C2H2. This is entirely consistent with the isotherms. C2H2 molecules at site I interact with bridging μ3-OH groups via πC2H2⋯H-O H-bond (2.96 Å) that is supplemented by weak C-H⋯Oligand H-bonding (dotted green lines, 2.75 Å, Fig. 4a and Supplementary Fig. 13b). CO2 molecules at site I are stabilized by C-OCO2⋯Hμ3-OH H-bonding (2.43 Å) and C-OCO2⋯Hligand interactions (2.80 and 3.64 Å, Fig. 4c and Supplementary Fig. 14b). C2H2 molecules at site II reside near the center of pore channels with fewer host-guest interactions (π⋯Hligand 3.31 Å), similar to that of CO2 in the channels at 298 K. However, CO2 molecules are located in the corner of the pore channels and stabilized by multiple weak host-guest interactions (C-OCO2⋯Hligand, 2.40–2.87 Å), thus leading to the strong binding affinities of host framework for CO2. Meanwhile, in Co-CUK-1, C2H2 clusters are formed with C2H2 molecules via πC2H2⋯πC2H2 interactions (dotted orange lines, 2.95 and 3.18 Å, Fig. 4b). The neighboring clusters synergistically interact with each other via weak C-H⋯π H-bonding (3.05 and 3.17 Å), leading to the efficient packing of C2H2 molecules. By contrast, the isolated CO2 clusters are formed with four CO2 molecules by closely interacting with each other (distances of 2.53 and 3.14 Å) with the head-to-center configurations, forming the quasi-T-shaped geometry (C = O⋯C, dotted azure lines, Fig. 4d). This is similar to that in dry ice, indicating the highly efficient packing of CO2 molecules (thus packing densities) in Co-CUK-1. Similar CO2 clusters with quasi-T-shaped dimers were also observed in Ni-CUK-1 at 233 K (Supplementary Fig. 16d). Thus, the notably stronger guest-guest interactions between adsorbed CO2 molecules at low temperature promote the unusually selective adsorption of CO2 over C2H2 at 233 K. Importantly, to the best of our knowledge, such guest-guest packing geometries and host-guest interactions at different temperatures have not been observed in porous materials yet.
To quantitatively compare the binding affinities of CUK-1 to CO2 and C2H2 at different temperatures, the static binding energies (ΔE) were further estimated by first-principles density functional theory (DFT) calculations (Supplementary Tables 2 and 3). The results show that after decreasing the temperature from 298 to 233 K, there are significant increases of ΔE at site I for CO2. Especially, ΔE for CO2 at site I on Co-CUK-1 is 43.5 kJ mol−1, much higher than that for C2H2 (33.3 kJ mol−1), directly validating the preferential adsorption of CO2 over C2H2. There is a subtle difference in host-guest interactions when varying the metal nodes in CUK-1 (Supplementary Tables 2 and 3), and this has little influence on the formation of guest clusters and the tunable CO2 and C2H2 sorption behavior. Thus, the guest-guest interactions and/or the arrangement of guest clusters play the dominant role in the inverse CO2 sorption of CUK-1 materials.
Dynamic breakthrough tests
Dynamic breakthrough experiments on CUK-1 materials using mixtures of CO2/C2H2 were conducted (Fig. 5). For the equimolar CO2/C2H2 mixture at 298 K, Ni- and Mg-CUK-1 show typical C2H2-preferential sorption over CO2 with a clear separation of C2H2 and CO2, but Co-CUK-1 shows very poor separation (Fig. 5a and Supplementary Fig. 19). These are consistent with the isotherm results at 298 K. At 273 K, a clear inversed CO2/C2H2 separation was observed on Co-CUK-1 (Fig. 5b and Supplementary Fig. 20), and there is an obvious deterioration in C2H2/CO2 separation performance on Ni- and Mg-CUK-1 (Supplementary Fig. 21). At 233 K, an evident inversed CO2/C2H2 separation was observed on CUK-1 materials, and all materials exhibit highly selective adsorption of CO2 over C2H2 (Fig. 5b, c). The dynamic CO2 uptake capacities at 233 K were calculated to be 140, 110, and 122 cm3 g−1 on Co-, Ni-, and Mg-CUK-1, respectively, much higher than those of C2H2 (62, 67, and 78 cm3 g−1, respectively). To mimic the industrial processes for C2H2 production, we further studied a gas mixture of CO2/C2H2 (1/2). A complete inversed CO2/C2H2 separation was realized with CO2 retained in the fixed bed for a longer duration (Fig. 5d). Significantly, the productivity of pure C2H2 (99.995%) can reach 62 and 41.7 L kg−1 on Co-CUK-1 and Mg-CUK-1, respectively, much higher than that on MUF-16 (27 L kg−1)39 and Cu-F-pymo (3.7 L kg−1)38. It is worth noting that these materials show excellent cycling separation performance and can be easily regenerated by purging helium (He) at 298 K (Supplementary Figs. 22–23). The notably high CO2 uptake and facile regeneration of CUK-1 are particularly desirable for practical applications to reduce the energy footprint compared with state-of-the-art cryogenic distillations.
a Breakthrough curves of CO2/C2H2 (1/1) mixture on CUK-1 materials at 298 K with a flow rate of 2.0 mL min−1. b Breakthrough curves of CO2/C2H2 (1/1) mixture on Co-CUK-1 at 273 and 233 K with flow rates of 2.0 and 3.0 mL min−1, respectively. c Breakthrough curves of CO2/C2H2 (1/1) mixture on Ni-CUK-1 and Mg-CUK-1 at 233 K with a flow rate of 3.0 mL min−1. d Breakthrough curves of CO2/C2H2 (1/2) mixture on CUK-1 materials at 233 K with a flow rate of 3.0 mL min−1.
Discussion
In summary, we report the direct observation of packing geometry rearrangement of gas clusters as a function of temperature to control the adsorption selectivity of CO2 and C2H2 on ultramicroporous MOFs. The strong host-guest interactions of CUK-1 for C2H2 at ambient conditions led to the preferential adsorption of C2H2. However, the efficient packing of CO2 molecules via strong guest-guest interactions forms CO2 clusters, leading to higher CO2 capacity. Impressively, after decreasing temperature, the host-guest interactions between CO2 and host framework became stronger than that for C2H2. Furthermore, the highly ordered arrangement of CO2 clusters with the T-shaped dimers endows CUK-1 with remarkably higher capacities for CO2 over those for C2H2. Such host-guest interactions, guest-guest interactions, and gas cluster formation were elucidated by in-situ synchrotron X-ray powder diffraction and molecular simulations. This idiosyncratic inversion of the adsorption behavior of C2H2 and CO2 was verified by dynamic breakthrough experiments with high-purity C2H2 (99.995%) obtained in a one-step process. Furthermore, the fixed-bed packed with CUK-1 can be easily regenerated at room temperature by purging an inert gas, indicating that the TSA process using CUK-1 materials is highly efficient for C2H2 production.
Methods
Gas adsorption and separation experiments
CO2 and C2H2 sorption isotherms were collected at different temperatures on a Micromeritics ASAP 2020 instrument equipped with commercial software for data calculation and analysis. The test temperatures were controlled by soaking the sample cell in a circulating water bath (298 K), ice/methanol mixture (233–273 K), dry ice/acetone mixtures (196 K), or liquid N2 (77 K). Before measurement, the sample (80–100 mg) was degassed at 423 K for 24 h. The breakthrough experiments were performed in a stainless-steel fixed bed (4.6 mm inner diameter × 50 mm length) packed with ~0.6 g of CUK-1 powder. Before the breakthrough experiment, the fixed bed was heated at 423 K under a flow of He for complete activation. The fixed bed was then cooled to room temperature and immersed in a water/methanol bath with different temperatures. Then, the gas mixtures (C2H2/CO2) were introduced, and the outlet gas was monitored by mass spectrometry (Hidden QGA quantitative gas analysis system).
In-situ synchrotron powder X-ray diffraction and structure determination
Fresh samples of Co-CUK-1 or Ni-CUK-1 were pre-activated under a dynamic vacuum at 200 °C, then loaded into a 0.7 mm borosilicate capillary under an inert atmosphere. Then the capillary was further activated by heating to 100 °C under a dynamic vacuum for 2 h before cooling down to room temperature. For samples measured under 233 K, synchrotron X-ray powder diffraction was carried out on the ID22 high-resolution powder diffraction beamline at the European Synchrotron Radiation Facility (ESRF). C2H2 or CO2 was introduced into the capillary, and diffraction data were collected after one-hour stabilization. Data were measured between 0 and 35° with a 13-channel multi-analyzer stage under the wavelength of 0.354267(4) Å. Data were binned using a step size of 0.002°. For samples measured under 298 K, high-resolution powder X-ray diffraction patterns were collected on the powder diffractometer (λ = 0.825829(1) Å) at room temperature on beamline I11 (Diamond Light Source, UK). C2H2 or CO2 was dosed offline and then sealed for measurement. Data were collected between 0 and 150° using a step size of 0.001° with five multi-analyzing crystal (MAC) detectors without further re-binned.
Pawley and Rietveld refinements of the structure were carried out using the TOPAS software package (roughly between 18–0.90 Å in d-spacing). Due to the high flexibility of the framework, index with constraints was used to get the information on cell parameters and space groups. Stephen fitting was applied to describe the diffraction peaks and their anisotropic broadening. Approximate positions of the guest molecule under a rigid body model were found using the simulated annealing approach before further refinement was used to find the optimal orientation of the guest molecules. The final refined structural parameters include cell parameters, the fractional coordinates (x, y, z) and isotropic displacement factors for all atoms except hydrogen, and the site occupancy factors (SOF) for guest molecules. The accuracy of the final model was verified by the convergence of the weighted profile factor (Rwp), the chemical sense of the model, and the good correlation between the observed and calculated diffraction patterns.
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2214437, 2214440–2214446. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All the other relevant data, additional graphics, and calculations that support the findings of this study are available within the article and its Supplementary Information, or from the corresponding authors upon request.
References
Beer, P., Barendt, T. A., Lim, J. Y. C. Supramolecular Chemistry: Fundamentals and Applications. (Oxford university press, 2022).
Lehn, J. M. Supramolecular chemistry: Where from? Where to? Chem. Soc. Rev. 46, 2378–2379 (2017).
Kolesnichenko, I. V. & Anslyn, E. V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 46, 2385–2390 (2017).
Yang, S. et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework. Nat. Chem. 7, 121–129 (2014).
Smith, G. L. et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metal-organic framework with open copper sites. Nat. Mater. 18, 1358–1365 (2019).
Shimomura, S. et al. Selective sorption of oxygen and nitric oxide by an electron-donating flexible porous coordination polymer. Nat. Chem. 2, 633–637 (2010).
Yoon, J. W. et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 16, 526–531 (2017).
Jaramillo, D. E. et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites. Nat. Mater. 19, 517–521 (2020).
Schneemann, A. et al. Flexible metal-organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Wang, S. et al. Toward a rational design of titanium metal-organic frameworks. Matter 2, 440–450 (2020).
He, T. et al. Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks. Nat. Mater. 21, 689–695 (2022).
Adil, K. et al. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 46, 3402–3430 (2017).
Ebadi Amooghin, A., Sanaeepur, H., Luque, R., Garcia, H. & Chen, B. Fluorinated metal-organic frameworks for gas separation. Chem. Soc. Rev. 51, 7427–7508 (2022).
Ji, Z., Wang, H., Canossa, S., Wuttke, S. & Yaghi, O. M. Pore chemistry of metal–organic frameworks. Adv. Funct. Mater. 30, 2000238 (2020).
Yang, Q., Xu, Q. & Jiang, H. L. Metal-organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis. Chem. Soc. Rev. 46, 4774–4808 (2017).
Bloch, E. D. et al. Hydrocarbon separations in a metal-organic framework with open iron (II) coordination sites. Science 335, 1606–1610 (2012).
Evans, J. D., Bon, V., Senkovska, I., Lee, H. C. & Kaskel, S. Four-dimensional metal-organic frameworks. Nat. Commun. 11, 2690 (2020).
Ma, Y. et al. A convenient strategy for designing a soft nanospace: an atomic exchange in a ligand with isostructural frameworks. J. Am. Chem. Soc. 137, 15825–15832 (2015).
Cui, X. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016).
Li, J. et al. Capture of nitrogen dioxide and conversion to nitric acid in a porous metal-organic framework. Nat. Chem. 11, 1085–1090 (2019).
Hu, J. & Liu, S. Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications. Acc. Chem. Res. 47, 2084–2095 (2014).
Islamoglu, T. et al. Metal-organic frameworks against toxic chemicals. Chem. Rev. 120, 8130–8160 (2020).
Zeng, H. et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 595, 542–548 (2021).
O’Keeffe, M. & Yaghi, O. M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 112, 675–702 (2012).
Liao, P. Q., Huang, N. Y., Zhang, W. X., Zhang, J. P. & Chen, X. M. Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017).
Wang, S. et al. A robust large-pore zirconium carboxylate metal–organic framework for energy-efficient water-sorption-driven refrigeration. Nat. Energy 3, 985–993 (2018).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal-organic microporous material. Nature 436, 238–241 (2005).
Schoedel, A., Li, M., Li, D., O’Keeffe, M. & Yaghi, O. M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 116, 12466–12535 (2016).
Chen, K. J. et al. Benchmark C2H2/CO2 and CO2/C2H2 separation by two closely related hybrid ultramicroporous materials. Chem. 1, 753–765 (2016).
Han, X. & Yang, S. Molecular mechanisms behind acetylene adsorption and selectivity in functional porous materials. Angew. Chem. Int. Ed. 62, e202218274 (2023).
Zhao, X., Wang, Y., Li, D. S., Bu, X. & Feng, P. Metal-organic frameworks for separation. Adv. Mater. 30, e1705189 (2018).
Li, J. R., Sculley, J. & Zhou, H. C. Metal-organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
Cui, W. G., Hu, T. L. & Bu, X. H. Metal-organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 32, e1806445 (2020).
Zhang, Z. et al. Efficient trapping of trace acetylene from ethylene in an ultramicroporous metal-organic framework: Synergistic effect of high-density open metal and electronegative sites. Angew. Chem. Int. Ed. 59, 18927–18932 (2020).
Gu, Y. et al. Host–guest interaction modulation in porous coordination polymers for inverse selective CO2/C2H2 separation. Angew. Chem. Int. Ed. 60, 11688–11694 (2021).
Niu, Z. et al. A MOF-based ultra-strong acetylene nano-trap for highly efficient C2H2/CO2 separation. Angew. Chem. Int. Ed. 60, 5283–5288 (2021).
Zhang, Z. et al. Optimal pore chemistry in an ultramicroporous metal-organic framework for benchmark inverse CO2/C2H2 separation. Angew. Chem. Int. Ed. 60, 17198–17204 (2021).
Shi, Y. et al. Highly selective adsorption of carbon dioxide over acetylene in an ultramicroporous metal-organic framework. Adv. Mater. 33, 2105880 (2021).
Qazvini, O. T., Babarao, R. & Telfer, S. G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 12, 197 (2021).
Ma, D. et al. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 8, 11933–11937 (2020).
Yoon, J. W. et al. Gas-sorption selectivity of CUK-1: a porous coordination solid made of cobalt(II) and pyridine-2,4- dicarboxylic acid. Adv. Mater. 19, 1830–1834 (2007).
Humphrey, S. M., Chang, J. S., Jhung, S. H., Yoon, J. W. & Wood, P. T. Porous cobalt (II)–organic frameworks with corrugated walls: Structurally robust gas‐sorption materials. Angew. Chem. Int. Ed. 119, 276–279 (2007).
Saccoccia, B. et al. Separation of p-divinylbenzene by selective room-temperature adsorption inside Mg-CUK-1 prepared by aqueous microwave synthesis. Angew. Chem. Int. Ed. 54, 5394–5398 (2015).
Mangan, T. P., Salzmann, C. G., Plane, J. M. C. & Murray, B. J. CO2 ice structure and density under Martian atmospheric conditions. Icarus 294, 201–208 (2017).
McIntosh, D. The physical properties of liquid and solid acetylene. J. Phys. Chem. 11, 306–317 (1907).
Foo, M. L. et al. An adsorbate discriminatory gate effect in a flexible porous coordination polymer for selective adsorption of CO2 over C2H2. J. Am. Chem. Soc. 138, 3022–3030 (2016).
Acknowledgements
This work was supported by the Ministry of Education Singapore (MOE2019-T2-1-093, MOE-T2EP10122-0002; D.Z.), the Energy Market Authority of Singapore (EMA-EP009-SEGC-020; D.Z.), the Agency for Science, Technology and Research (U2102d2004, U2102d2012; D.Z.), the National Research Foundation (NRF-CRP26-2021RS-0002; D.Z.), EPSRC (EP/V056409/1; S.Y.), and the University of Manchester. We are grateful to Diamond Light Source and European Synchrotron Radiation Facility (ESRF) for access to Beamlines I11 and ID22, respectively. X.H. is supported by a Dame Kathleen Ollerenshaw Fellowship.
Author information
Authors and Affiliations
Contributions
D.Z. and S.Y. formulated and supervised the project. Z.Z. synthesized and characterized the materials, measured the adsorption isotherms and the breakthrough curves, and wrote the manuscript. Y.C., X.H., C.D., S.J.D., and S.Y. collected and analyzed the in-situ synchrotron X-ray diffraction data. K.C. and C.K. helped collect the dynamic breakthrough data. S.P., H.L., J.R., and X.S. contributed to the data analysis and discussion. All authors contributed to the manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Nyasha Makuve, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Chen, Y., Chai, K. et al. Temperature-dependent rearrangement of gas molecules in ultramicroporous materials for tunable adsorption of CO2 and C2H2. Nat Commun 14, 3789 (2023). https://doi.org/10.1038/s41467-023-39319-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39319-2
- Springer Nature Limited
This article is cited by
-
Switching molecular recognition selectivities by temperature in a diffusion-regulatory porous material
Nature Communications (2024)