Abstract
A growing body of research suggests that social or physical activity can affect the risk of Major depressive disorder (MDD). However, the bidirectional relationship between them remains to be clarified further, especially between inactivity and MDD. Here, we performed a two-sample Mendelian Randomization analysis using genetic variants associated with social/physical activities and MDD, and assessed the mediating effect of obesity-related measures and brain imaging phenotypes. The dataset on MDD, social activities, and physical activities included 500,199; 461,369; 460,376 individuals, respectively. Information regarding body mass index (BMI), body fat percentage (BFP), IDPs for 454,633; 461,460; 8,428 participants, respectively. We identified bidirectional causal relationships between sport clubs or gyms, strenuous sports, heavy do-it-youself, other exercises and MDD. We also observed that leisure/social inactivity (odds ratio [OR] = 1.64; P = 5.14 × 10−5) or physical inactivity (OR = 3.67; P = 1.99 × 10−5) caused an increased risk of MDD, which were partially mediated by BMI or BFP and masked by the weighted-mean orientation dispersion index of left acoustic radiation or volume of right caudate. Furthermore, we discovered that MDD increased the risk of leisure/social inactivity (OR = 1.03; P = 9.89 × 10−4) or physical inactivity (OR = 1.01; P = 7.96 × 10−4). In conclusions, we found that social/physical activities reduced the risk of MDD, while MDD in turn hindered social/physical activities. Inactivity may increase the risk of MDD, which was mediated or masked by brain imaging phenotypes. These results help to understand the manifestations of MDD and provide evidence and direction for the advancement of intervention and prevention.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a common medical condition that severely lowers the quality of life and limits psychosocial functioning [1]. MDD is a leading cause of disability and plays an important role in the global burden of disease [2]. The etiology of MDD includes both physiological and social factors. In contrast with factors that increase MDD risk such as poverty, unemployment, family history, illness, and life events [2, 3], leisure/social activities and physical activities have received increasing attention as relatively easy-to-control risk factors [4,5,6].
Meta-analyses of prospective cohort studies showed that physical activities are associated with a reduced risk of MDD, regardless of age and geographical region [5, 7]. Other studies have indicated that leisure activities or good social relationships are related to lower depressive symptoms or a lower prevalence of MDD [8,9,10]. Symptoms of MDD can also include loss of energy and interest in previously enjoyed activities, which may lead to decreased social or physical participation [11]. This provides evidence of a bidirectional causal relationship between social/physical activity and MDD which could not be ascertained through traditional observational studies [6, 12]. Considering that physical activity is often related to the prevention and management of obesity [13, 14], obesity traits could be mediators of the effect of activity on MDD.
Mendelian Randomization (MR) is an epidemiological method used to infer possible causal relationships between exposure and outcome by introducing single nucleotide polymorphisms (SNPs) associated with exposure (such as physical activity) as instrumental variables (IVs) [15]. As alleles follow the principle of random segregating combinations, the causal effect is theoretically unperturbed by confounding factors and reverse causes [16]. IVs can be extracted from publicly available genome-wide association studies (GWAS) summary statistics, which are widely used for two-sample MR [17]. A previous MR study showed a protective relationship between accelerometer-based activity and MDD [6]. An exposure-wide MR analysis assessed bidirectional causality between more than 100 modifiable factors (including those of social and physical activity) and MDD [18]. However, physical activity GWAS could include inactivity, and the relationship between inactivity and MDD remains unexplored. Guo et al. showed a causal relationship between brain phenotype and psychiatric disorders [12]. The brain imaging-derived phenotypes (IDPs) may play a mediating role between inactivity and MDD. Hence, an analysis of this aspect will help to prevent the risks and understand the mechanism between inactivity and MDD.
In this study, we aimed to examine the bidirectional causal effects between MDD and different types of leisure/social activity, physical activity, or “none of the above” (leisure/social inactivity or physical inactivity). We also assessed the mediating effect of obesity-related measures and IDPs on the association between different types of activities or inactivity and MDD.
Methods
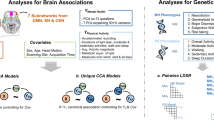
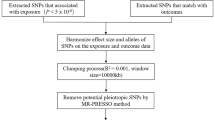
This two-sample MR was based on data from publicly available GWAS summary statistics, and ethical approval was obtained from the original studies. Details of the data sources used in this study are summarized in Supplementary Table S1. We extracted leading SNPs associated with social/physical activities and MDD as genetic IVs to perform bidirectional two-sample MR (Supplementary Fig. S1). Moreover, two-step MR analyses were used to assess whether obesity metrics and IDPs play a role as mediators between inactivity and MDD (Fig. 1). In addition, to minimize racial mismatches, the current analyses were restricted to participants of European descent.
To filter eligible genetic instruments, we performed a series of quality controls. First, we included SNPs that were strongly associated with exposure factors with P < 5 × 10−8. Second, we conducted clumping with R2 < 0.001 and a window size of 10,000 kb. Third, proxy SNPs (R2 > 0.8) replaced the SNPs of exposure when the targeted SNPs were not found in the outcome datasets. Fourth, the minor allele frequency of SNPs found in the outcome GWAS was set to a default of 0.3. Finally, we harmonized the exposure and outcome to eliminate palindromic SNPs and ensure that the effect alleles belonged to the same allele [19]. We also investigated each IV in the PhenoScanner GWAS database [20] and removed the SNPs associated with plausible confounders (such as alcohol consumption and smoking) [21]. The application of less than 10 independent SNPs as IVs may decrease statistical efficiency in MR analysis [22]. Therefore, we use a relaxed and previously used instrument threshold (P < 1 × 10−5) [23] to ensure the number of SNPs, when traits lacked sufficient SNPs (≤10).
Furthermore, to quantify the strength of IVs, we calculated the F-statistics of all genetic instruments using the genetic variants (R2), sample size (N), and the number of instruments (k) using the formula F = R2(N-k-1)/k(1-R2) [24]. When the F-statistic was less than 10, the genetic variant was considered weak, which may bias the results [25].
We utilized publicly available GWAS summary-level data associated with leisure/social activities, including sport clubs or gyms, pubs or social clubs, religious groups, adult education classes, other group activities, and “none of the above” (leisure/social inactivity), from the Medical Research Council Integrative Epidemiology Unit (MRC-IEU) UK biobank (Supplementary Table S1) entailing 461,369 participants. An individual who selected “none of the above” on the leisure/social activities questionnaire means that he or she participated in leisure/social activities less than once a week in the last year, which we call “leisure/social inactivity”. Genetic variants associated with types of physical activity in the last 4 weeks from the MRC-IEU were also used to identify the instruments. The questionnaire on types of physical activities included walking for pleasure (not as a means of transport), strenuous sports (sports that make you sweat or breathe hard), light “do-it-yourself” (DIY; such as pruning and watering the lawn), heavy DIY (such as weeding, lawn mowing, carpentry, and digging), other exercises (such as swimming, cycling, keeping fit, bowling), and “none of the above” (physical inactivity), consisting of 460,376 individuals (Supplementary Table S1). Choosing “none of the above” in this questionnaire means refusing any form of physical activity for the past four weeks, which we call “physical inactivity”. The summary information of IVs was showed in the Supplementary Tables S2–S13.
Summary statistics for MDD were obtained from a meta-analysis of GWAS involving 170,756 cases and 329,443 controls of European descent, which included participants from the Psychiatric Genomics Consortium (PGC) and UK Biobank (excluding 23andme) [26]. When the genetic variables associated with MDD were used as instruments, a total of 49 SNPs were identified to be significantly associated with MDD (P < 5 × 10−8), and 47 SNPs satisfied the standard for IVs (Supplementary Table S14). For avoid overlapping sample, when MDD was outcome, we used the other GWAS summary data for MDD removed UK Biobank and 23andMe samples to conduct a series of subgroup analyses, which included subsample of 143,265 (45,591 cases and 97,674 controls) [27]. The IVs associated with the potential confounders are listed in Supplementary Table S15.
We used publicly available GWAS summary statistics of potential mediators (Supplementary Table S1), including body fat percentage (BFP) in 454,633 individuals, body mass index (BMI) in 461,460 samples, hip circumference (HP) in 462,117 individuals, waist circumference (WC) in 462,166 participants from MRC-IEU [28], and waist-to-hip ratio (WHR) in 212,244 individuals from the Genetic Investigation of ANthropometric Traits (GIANT) [29]. For brain imaging phenotypes, we used the summary data of 3144 functional and structural brain IDPs from UK Biobank (discovery dataset 8428 subjects) [30], which covered the whole brain and including multimodal information on white matter connections, functional connectivity, grey matter volume, area and thickness. The summary statistics of IDP were available from the Oxford Brain Imaging Genetics (BIG) web browser (http://big.stats.ox.ac.uk/) [30].
Statistical analyses
All analyses were conducted using the TwoSampleMR R package [31]. Our primary MR method was inverse-variance weighted (IVW) regression [32], which is currently common and easy 2-sample MR method and often is used as major analysis method in many studies [18, 33, 34]. In addition, we also adopted complementary methods, including the MR-Egger regression, weighted median, simple mode, and weighted mode, to estimate the causal effects of exposure on outcomes [35,36,37]. A detailed description of these methods is attached in the Supplementary methods.
Next, we performed a series of sensitivity analyses to assess the robustness and pleiotropy of causal estimates. We used a random-effect modal IVW MR analysis when Cochran’s Q statistic suggested the heterogeneity of different genetic variants [38]. We also performed MR-egger intercept test for directional pleiotropy, which would be proved when the intercept term deviated from zero (P < 0.05) [39, 40]. A leave-one-out IVW regression was used to test the robustness of the results. In addition, we utilized the Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis [41] to detect the outlier SNPs reflecting likely pleiotropy with a P value of 0.05, and correct for horizontal pleiotropy by removing the outlier. Associations with P values below 0.004 (0.05/12) were deemed statistically significant evidence of a causal association. P value between 0.004 and 0.05 was regarded as evidence of potential causal association [23]. Additionally, MR estimates could be subject to different types of biases due to the overlap between the exposure and outcome samples [42]. Thus, we used the MRlap R package to account and correct biases due to the overlapping samples (see supplementary methods) [43].
For all the significant association MR results of different activities on MDD, we conducted two-step MR analyses to detect the potential mediating effects of obesity metrics. When the exposures influenced the mediator, which in turn influenced MDD risk, we used the “product of coefficients” [44]. In addition, for inactivity on MDD, 3144 functional and structural IDPs were regarded as mediators to conduct two-step MR (Fig. 1). We extracted IDPs where both steps MR were significant (P < 0.05) and overlapping as mediators and examined the significance of the heritability of these IDPs.
In the current analysis, we expressed our MR results as odds ratios (ORs) accompanied by their respective 95% confidence intervals (CIs), which represent the outcome risk associated with unit changes in exposure. Since all exposure variables in this study are binary, we interpreted the final effect estimates as ORs on the outcome risk per log-OR change in exposure [45].
Results
Causal effects of different activities on MDD
After removing outliers, MR analysis showed a protective causal relationship between attending sports clubs or gyms. Per log-OR increment in attending sports clubs or gyms was associated with 49% lower risk of MDD (IVW OR = 0.51; 95% CI, 0.39 to 0.67; P = 1.05 × 10−6) and MDD (Fig. 2). However, the OR for MDD of genetically predicted per log-OR increase in leisure/social inactivity was 1.64 (95% CI, 1.29 to 2.08; P = 5.14 × 10−5) (Fig. 3, Supplementary Table S16 and Figures S2, S3). There was no statistically significant difference in the rest of the leisure/social activities.
After removal of outliers (Fig. 2, Supplementary Table S17 and Figures S2, S3), MR analysis identified protection from physical activities of walking for pleasure (IVW OR = 0.13; 95% CI, 0.07 to 0.26; P = 4.25 × 10−9), strenuous sports (IVW OR = 0.43; 95% CI, 0.27 to 0.71; P = 9.16 × 10−4), light DIY (IVW OR = 0.47; 95% CI, 0.32 to 0.68; P = 9.65 × 10−5), Heavy DIY (IVW OR = 0.51; 95% CI, 0.35 to 0.76; P = 7.46 × 10−3), or other exercises (IVW OR = 0.44; 95% CI, 0.35 to 0.55; P = 3.30 × 10−12) on MDD. However, per log-OR increase in physical inactivity was associated with a 3.67-fold increase in the risk of MDD (IVW OR = 3.67; 95% CI, 2.02 to 6.68; P = 1.99 × 10−5; Fig. 3). All MR-Egger intercept tests indicated no horizontal pleiotropy. SNPs exclusion demonstrated the stability of the results (Supplementary Fig. S4). The F-statistics found no weak instrument bias. Apart from walking for pleasure and light DIY, the results of the subgroup analyses without overlapping samples remained significant (for detail see Supplementary Table S18). The results of MRlap showed the differences between observed and corrected effects were not significant (Supplementary Table S19).
Causal effects of MDD on different activities
In the other direction, genetic liabilities for MDD were used as exposures. After excluding outliers, the MR results showed a protective causal relationship between MDD and sport clubs or gyms (IVW OR = 0.96; 95% CI, 0.95 to 0.98; P = 1.82 × 10−6), but the OR of leisure/social inactivity of genetically predicted per log-OR increase in MDD was 1.03 (95% CI, 1.01 to 1.04; P = 9.89 × 10−4; Figs. 2, 3, Supplementary Table S20 and Figs. S5, S6). Results were not statistically significant for the effect of MDD on the rest of leisure/social activities.
With genetic variants for different types of physical activities as outcomes, the causal estimates from MDD to heavy DIY (IVW OR = 0.97; 95% CI, 0.96 to 0.99; P = 1.54 × 10−3) or other exercises (IVW OR = 0.96; 95% CI, 0.94 to 0.98; P = 1.88 × 10−6) were significant, after removing outliers. MDD was associated with lower likelihood of attending strenuous sports (IVW OR = 0.99; 95% CI, 9.79 × 10−1 to 9.99 × 10−1; P = 4.41 × 10−2). In addition, MR also revealed a causal relationship between MDD and physical inactivity (IVW OR = 1.01.; 95% CI, 1.01 to 1.02; P = 7.96 × 10−4) (Fig. 3). The MR results showed no significant effects of MDD on light DIY or walking for pleasure, even after removing outliers (Supplementary Table S21). The F-statistics found no weak instrument bias. Furthermore, all the MR-Egger intercept tests indicated no horizontal pleiotropy. The analyses excluding each SNP indicated that no single SNP drove these results (Supplementary Fig. S7).
Mediation analysis
We conducted a two-step MR analysis to investigate a causal relationship between the different activity types and MDD through obesity-related metrics. First, instruments for different types of activities were used to estimate the causal relationship between exposure and potential mediators. Across all types of activities and the mediators, we identified significant causal effects (Supplementary Tables S22–S26). Second, genetic instruments for obesity-related measurements were used to assess the causal effect of mediators on MDD risk. We found a relationship between BFP, BMI, HC, or WC and MDD (Supplementary Table S27). Given that obesity-related measurements are related to each other, we conducted a multivariable MR analysis to estimate the direct effect of each on MDD. Among the potential mediators, only BFP (OR = 1.34; 95% CI, 1.07 to 1.67; P = 0.01) and BMI (OR = 1.23; 95% CI, 1.02 to 1.50; P = 0.03) reached significance (Supplementary Table S28). Finally, we estimated the indirect effect of the types of activity on MDD via BFP or BMI (Supplementary Table S29). For leisure/social inactivity, we found the mediation effect of BFP was 0.06 (95% CI, 0.02 to 0.12) with a mediated proportion of 12.99% (Table 1 and Fig. 4) and the mediation effect of BMI was 0.06 (95% CI, 0.02 to 0.11) with a mediated proportion of 11.80%. The mediation effects of BFP and BMI were 0.23 (95% CI, 0.11 to 0.40) and 0.19 (95% CI, 0.08 to 0.34) between physical inactivity and MDD, and the effect proportions were 17.82% and 14.38% respectively.
‘A’ indicates two-step MR results of leisure/social inactivity on MDD. ‘B’ indicates two-step MR results of physical inactivity on MDD. BFP, body fat percentage; BMI, body mass index; CI, confidence interval; IVW, inverse variance weighted method; MDD, major depressive disorder; MR, Mendelian Randomization; Volume of GM in RHG, volume of grey matter in right heschl’s gyrus; Weighted-mean OD in LAR, weighted-mean orientation dispersion index in tract left acoustic radiation; Volume of RC, volume of right caudate; Area of LPG, area of precentral gyrus in the left hemisphere; Mean OD in RSS, mean orientation dispersion index in right sagittal stratum; Mean ISOVF in RSCR, mean isotropic or free water volume fraction in right superior corona radiata.
In addition, 3144 IDPs were used as mediators to examine the mediating pathway between inactivity and MDD. First, instruments for inactivity were used to estimate the causal relationship between inactivity and brain imaging phenotypes. Across all 3144 IDPs, we identified significant causal effects from leisure/social inactivity or physical inactivity to IDPs (Supplementary Tables S30, S31). Second, genetic instruments for IDPs were used to assess the causal effect of mediators on MDD risk (Supplementary Table S32). And then, the significant and overlapping IDPs in both steps MR were extracted and examined their heritability (Supplementary Table S33). Finally, only seven IDPs met the criteria (Fig. 4). The mediation analyses showed the relationship of leisure/social inactivity on MDD was partly mediated by volume of grey matter in right heschl’s gyrus (4.03%) and volume of third ventricle (5.01%). Due to the indirect and direct effects of weighted-mean orientation dispersion index (OD) of left acoustic radiation showing opposite signs, we concluded it as masking effect [46], and the effect proportion was 8.43%. For the physical inactivity on MDD, we found the partial mediation effect of the surface area in left precentral gyrus (3.29%), mean OD in right sagittal stratum (4.05%), and mean isotropic or free water volume fraction (ISOVF) in right superior corona radiata (3.86%). The volume of right caudate has a masking effect with a effect proportion of 4.74% (Table 1).
Discussion
In this MR study, significant potential causal effects between different types of leisure/social or physical activities and MDD were identified. We detected protective effects from attending sports clubs or gyms, strenuous sports, heavy DIY and other exercises. In contrast, MR results showed a significant potential causal relationship between MDD and reduced leisure/social or physical activities, such as sport clubs or gyms attendance, strenuous sports, heavy DIY, and other exercises. Unlike previous study [18], we focused on types of activity, particularly inactivity. We observed bidirectional causal effect between recent leisure/social or physical inactivity and MDD. In addition, we focused on the risk mechanisms of inactivity and found that the effects of activity or inactivity on MDD are partially mediated by BFP and BMI. Some IDPs played a mediating or masking role between inactivity and MDD. Our results proved robust after a series of sensitivity analyses and pleiotropy assessment.
Our study showed a significant protective causal effect from attending sports clubs or gyms to MDD. Specifically, Kleppang et al. suggested attending sports club was associated significantly with lower odds for symptoms of MDD, which means social activity might play a vital role in addition to physical activity [47]. Attending leisure/social activities also often entail a certain amount of physical activity, and our work showed that physical activity is protective against MDD. However, a prior MR study suggested that the causal relationship between self-reported activity and MDD is not significant [6], perhaps owing to the differences in the strength of genetic instruments or self-report measures. In the current study, majority MR results from physical activity types to MDD were significant, which provide that physical activities are effective in preventing MDD [5]. This is important for public health. A previous study also showed that approximately 12% of MDD cases could be prevented through an hour of physical activity every week [48].
We also aimed to elucidate the direction of the estimated causal effects between physical/social activities and MDD. Through bidirectional MR, we found that patients with MDD may lead less likely to attend sport clubs or gyms, strenuous sports, heavy DIY, and other exercises, although this effect is not great. These are consistent with the symptoms of MDD, such as loss of energy and interest in previously enjoyed activities [11]. Another study also showed that depressive symptoms were associated with sedentariness and reduced physical activity [49]. Interestingly, sport clubs or gyms, strenuous sports, heavy DIY, and other exercises all showed evidence of a bidirectional causality; that is, attending these activities reduced the risk of MDD, but MDD also reduced the occurrence of these events.
To our knowledge, this study is the first to investigate the potential bidirectional causal relationship between social/physical inactivity and MDD using the MR method. The protective effects of social activities and larger social networks in MDD have been demonstrated [4, 18]. As an extension, we explored the causal relationship between not participating in leisure/social activities and MDD. In addition, a previous study has shown that people who participated less in sport clubs or physical/social activities exhibited higher odds of symptoms of MDD [50]. In this case, the physical aspect could trump the social one. Our results also suggest that physical inactivity may increase the risk of MDD. Inactivity in the last 4 weeks often indicated a lack of exercise or sedentary behavior. Sedentary behavior is slightly different from lack of exercise, though they have both been significantly associated with MDD and anxiety [51]. One study [52] indicated that mentally passive sedentary behaviors (such as watching TV or sitting around) increased the risk of MDD, whereas mentally active sedentary behaviors (such as reading or driving) do not increase the risk of MDD [52]. Conversely, MDD increased the risk of social/physical inactivity, which might lead to a sedentary lifestyle [49, 53]. There seems to be a vicious circle in which inactivity increases the risk of MDD, and MDD itself may be a reason for avoidance of social/physical activities. Physiological links between a lack of activity and MDD include hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system hyperactivity, increased inflammatory markers, and lower pain thresholds [53]. Further investigation is needed to determine how these underlying mechanisms may contribute to the potential bidirectional causality.
Two-step MR for mediation analysis showed that the protective effect of leisure/social or physical activities was partially mediated by decreased BFP and BMI, and that inactivity increased the risk of MDD in part by raising BFP and BMI. A recent MR study reported that moderate-vigorous physical activities lower the BMI, and longer sedentary time increases the BMI [54], which is consistent with the first-step MR. MR studies showed causal risk factors for BMI or BFP in MDD [55, 56], which agrees with our findings. Interestingly, when the IDPs were regarded as mediators, we found that leisure/social inactivity increased the weighted-mean OD in tract left acoustic radiation, which in turn leads to a reduction of risk of MDD. In other words, the presence of increased weighted-mean OD in tract left acoustic radiation seems to mitigate the total effects of leisure/social inactivity on MDD. Similarly, volume of right caudate played a masking role between physical inactivity and MDD, which means the decreased volume of the right caudate may attenuate the effect of physical inactivity on MDD [46]. Besides, studies also showed that exercise could reshape the brain structure of MDD patients and maintain the integrity of hippocampal and white matter, which helped promote behavioral adaption changes [57].
Our study had some limitations. First, although strongly associated SNPs were selected, they cannot be considered exact proxies for exposure [58]. Additionally, we also could not determine whether physical activity in the past 4 weeks is representative of long-term lifestyle habits of individuals. The MDD metric used in our study measures long-term effects, and further research could differentiate between first-episode depression and relapse to better showcase the risk associated with inactivity. Second, our study relied primarily on self-reporting, which may not be able to exclude emotional states and cognitive biases that can also influence mental health. Although this does not diminish the usefulness of self-reported measures, the accelerometer-based activity study by Choi et al. [6] also suggests that we should consider objectively measured actigraphy data in future studies to consider inactivity in a more accurate manner. Third, UK Biobank summary data was used in this study only included European ancestry population, hence, whether the results can be generalized to other populations remains to be verified. Moreover, we were not able to determine whether gender would bias the results due to the summary data lacking sufficient information. Fourth, in order to obtain sufficient SNPs and ensure the accuracy of the results, we sometimes had to relax the threshold. It is difficult to completely eliminate potential pleiotropy due to lack of knowledge of potential confounders and inability to get individual level data. [59]. Nevertheless, we inferred robust causal effects by outlier removal using MR-PRESSO and a series of sensitivity analyses. Fifth, there were likely overlapping samples in the exposure and outcome studies, which could have resulted in weak instrument bias. To prevent this, we used strong instruments (F statistic much greater than 10) [60], subgroup analysis without overlapping samples, and MRlap approach. Finally, we used binary variables in the study, which led to our inability to determine more specific risk effect parameters (such as time and frequency of activity). Additionally, although some MR results were significant when MDD was used as an exposure, the effect sizes were small and more studies are needed to validate them.
Conclusions
We found potential bidirectional causal relationships between sports clubs or gyms, strenuous sports, heavy DIY, other exercises and MDD. Social or physical activity reduced the risk of MDD, but MDD was a cause of reduced social and physical activity. This bidirectional causal effect between social or physical inactivity and MDD may be evidence of a vicious cycle that may account for the steady increase in the burden of MDD. These results provided support for the development of novel prevention and intervention strategies for MDD. In addition, the mediating effect of obesity-related measures or IDPs provided more information to understand the mechanism of inactivity on MDD.
Data availability
All GWAS summary statistics analyzed in this study are publicly available for download by qualified researchers.
Code availability
All the analyses used in this study were performed R packages TwoSampleMR (version 0.5.6), MR-PRESSO (version 1.0) and MRlap (version 0.0.3.0). The code is available at https://mrcieu.github.io/TwoSampleMR/ and https://github.com/n-mounier/MRlap.
References
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Friedrich MJ. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317:1517.
Hammen C. Risk Factors for Depression: An Autobiographical Review. Annu Rev Clin Psychol. 2018;14:1–28.
Santini ZI, Koyanagi A, Tyrovolas S, Mason C, Haro JM. The association between social relationships and depression: a systematic review. J Affect Disord. 2015;175:53–65.
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am J Psychiatry. 2018;175:631–48.
Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2019;76:399–408.
Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:550–59.
Nagata S, McCormick B, Piatt J. Leisure behavior and sense of coherence in the context of depression. J Community Psychol. 2020;48:283–301.
Schuch FB, Werneck AO, Vancampfort D, Stubbs B, Teychene M, Lotufo PA, et al. Cross-sectional associations of leisure and transport related physical activity with depression and anxiety. J Psychiatr Res. 2021;140:228–34.
Kaveladze B, Diamond Altman A, Niederhausen M, Loftis JM, Teo AR. Social relationship quality, depression and inflammation: A cross-cultural longitudinal study in the United States and Tokyo, Japan. Int J Soc Psychiatry. 2022;68:253–63.
Association AP Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
Guo J, Yu K, Dong SS, Yao S, Rong Y, Wu H, et al. Mendelian randomization analyses support causal relationships between brain imaging-derived phenotypes and risk of psychiatric disorders. Nat Neurosci. 2022;25:1519–27.
Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw KT, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 2010;7:e1000332.
Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond). 2018;42:1161–76.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 2018;362:k601.
Porcu E, Rüeger S, Lepik K, Santoni FA, Reymond A, Kutalik Z. Mendelian randomization integrating GWAS and eQTL data reveals genetic determinants of complex and clinical traits. Nat Commun. 2019;10:3300.
Choi KW, Stein MB, Nishimi KM, Ge T, Coleman JRI, Chen CY, et al. An Exposure-Wide and Mendelian Randomization Approach to Identifying Modifiable Factors for the Prevention of Depression. Am J Psychiatry. 2020;177:944–54.
Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–26.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019;35:4851–53.
Kim H, Yoo J, Han K, Fava M, Mischoulon D, Park MJ, et al. Associations Between Smoking, Alcohol Consumption, Physical Activity and Depression in Middle-Aged Premenopausal and Postmenopausal Women. Front Psychiatry. 2021;12:761761.
Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun. 2018;9:224.
Lu Z, Pu C, Zhang Y, Sun Y, Liao Y, Kang Z, et al. Oxidative Stress and Psychiatric Disorders: Evidence from the Bidirectional Mendelian Randomization Study. Antioxid (Basel). 2022;11:1386.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Elsworth BMR, Raistrick C, Paternoster L, Hemani G, Gaunt T MRCIEU UK Biobank GWAS pipeline version 1. 105523/BRIS2FAHPKSONT1ZI26XOSYAMQO8RR. 2017.
Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–96.
Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 2018;562:210–16.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65.
Yao S, Zhang M, Dong SS, Wang JH, Zhang K, Guo J, et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav. 2022;6:1569–76.
Yin KJ, Huang JX, Wang P, Yang XK, Tao SS, Li HM, et al. No Genetic Causal Association Between Periodontitis and Arthritis: A Bidirectional Two-Sample Mendelian Randomization Analysis. Front Immunol. 2022;13:808832.
Bowden J, Davey, Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304–14.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36:1783–802.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, et al. Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. Eur Heart J. 2021;42:2816–23.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–98.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608.
Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol. 2023;47:314–31.
VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32.
Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33:947–52.
Wen Z, Ye B. Analyses of Mediating Effects: The Development of Methods and Models. Adv Psychological Sci. 2014;22:731–45.
Kleppang AL, Hartz I, Thurston M, Hagquist C. The association between physical activity and symptoms of depression in different contexts - a cross-sectional study of Norwegian adolescents. BMC Public Health. 2018;18:1368.
Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am J Psychiatry. 2018;175:28–36.
Wu IHC, Strong LL, Nguyen NT, Cho D, John J, McNeill LH. Psychosocial Stressors, Depression, and Physical Activity among African Americans. Am J Health Behav. 2019;43:717–28.
Kleppang AL, Skille E. Leisure-Time Activities in Different Contexts and Depressive Symptoms in Norwegian Adolescents: A Cross-Sectional Study. Int J Environ Res Public Health. 2022;19:10769.
Bélair MA, Kohen DE, Kingsbury M, Colman I. Relationship between leisure time physical activity, sedentary behaviour and symptoms of depression and anxiety: evidence from a population-based sample of Canadian adolescents. BMJ Open. 2018;8:e021119.
Huang Y, Li L, Gan Y, Wang C, Jiang H, Cao S, et al. Sedentary behaviors and risk of depression: a meta-analysis of prospective studies. Transl Psychiatry. 2020;10:26.
Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–15.
Carrasquilla GD, García-Ureña M, Fall T, Sørensen TIA, Kilpeläinen TO. Mendelian randomization suggests a bidirectional, causal relationship between physical inactivity and adiposity. Elife 2022;11:e70386.
Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD. Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry. 2019;9:184.
Casanova F, O’Loughlin J, Martin S, Beaumont RN, Wood AR, Watkins ER, et al. Higher adiposity and mental health: causal inference using Mendelian randomization. Hum Mol Genet. 2021;30:2371–82.
Zhao JL, Jiang WT, Wang X, Cai ZD, Liu ZH, Liu GR. Exercise, brain plasticity, and depression. CNS Neurosci Ther. 2020;26:885–95.
Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14:507–15.
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195–r208.
Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013;178:1177–84.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81825009); National Key R&D Program of China (2021YFF1201103); Academy of Medical Sciences Research Unit (2019-I2M-5-006); Chinese Institute for Brain Research at Beijing (2020-NKX-XM-12); PKUHSC-KCL Joint Medical Research (BMU2020KCL001). The funders did not have any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. We would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Contributions
Conceptualization, GZ, ZL and WY; data curation, GZ, ZL, and YZ; formal analysis, GZ; funding acquisition, WY; investigation, WY; methodology, GZ, ZL, ZK and XF; project administration, WY; resources, WY; software, ZL, YS, YH; supervision, WY; visualization, GZ, YL, JS and ZL; writing—original draft, GZ; writing—review and editing, ZL, YZ, HY and WY. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, G., Lu, Z., Sun, Y. et al. Dissecting the causal association between social or physical inactivity and depression: a bidirectional two-sample Mendelian Randomization study. Transl Psychiatry 13, 194 (2023). https://doi.org/10.1038/s41398-023-02492-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02492-5
- Springer Nature Limited