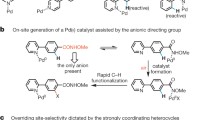
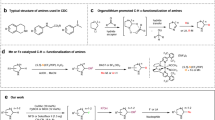
Abstract
Nitrogen-rich heterocyclic compounds have had a profound effect on human health because these chemical motifs are found in a large number of drugs used to combat a broad range of diseases and pathophysiological conditions. Advances in transition-metal-mediated cross-coupling have simplified the synthesis of such molecules; however, C–H functionalization of medicinally important heterocycles that does not rely on pre-functionalized starting materials is an underdeveloped area1,2,3,4,5,6,7,8,9. Unfortunately, the innate properties of heterocycles that make them so desirable for biological applications—such as aqueous solubility and their ability to act as ligands—render them challenging substrates for direct chemical functionalization. Here we report that zinc sulphinate salts can be used to transfer alkyl radicals to heterocycles, allowing for the mild (moderate temperature, 50 °C or less), direct and operationally simple formation of medicinally relevant C–C bonds while reacting in a complementary fashion to other innate C–H functionalization methods2,3,4,5,6 (Minisci, borono-Minisci, electrophilic aromatic substitution, transition-metal-mediated C–H insertion and C–H deprotonation). We prepared a toolkit of these reagents and studied their reactivity across a wide range of heterocycles (natural products, drugs and building blocks) without recourse to protecting-group chemistry. The reagents can even be used in tandem fashion in a single pot in the presence of water and air.


Similar content being viewed by others
References
Brückl, T., Baxter, R. D., Ishihara, Y. & Baran, P. S. Innate and guided C–H functionalization logic. Acc. Chem. Res. 45, 826–839 (2012)
Minisci, F., Vismara, E. & Fontana, F. Recent developments of free-radical substitutions of heteroaromatic bases. Heterocycles 28, 489–519 (1989)
Duncton, M. A. J. Minisci reactions: versatile CH-functionalizations for medicinal chemists. Med. Chem. Comm. 2, 1135–1161 (2011)
Seiple, I. B. et al. Direct C–H arylation of electron-deficient heterocycles with arylboronic acids. J. Am. Chem. Soc. 132, 13194–13196 (2010)
Fujiwara, Y. et al. Practical C–H functionalization of quinones with boronic acids. J. Am. Chem. Soc. 133, 3292–3295 (2011)
Molander, G. A., Colombel, V. & Braz, V. A. Direct alkylation of heteroaryls using potassium alkyl- and alkoxymethyltrifluoroborates. Org. Lett. 13, 1852–1855 (2011)
Ji, Y. et al. Innate C–H trifluoromethylation of heterocycles. Proc. Natl Acad. Sci. USA 108, 14411–14415 (2011)
Langlois, B. R., Laurent, E. & Roidot, N. Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions. Tetrahedr. Lett. 32, 7525–7528 (1991)
Fujiwara, Y. et al. A new reagent for direct difluoromethylation. J. Am. Chem. Soc. 134, 1494–1497 (2012)
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001)
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011)
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008)
Kirk, K. L. Fluorination in medicinal chemistry: methods, strategies, and recent developments. Org. Process Res. Dev. 12, 305–321 (2008)
Ma, J.-A. & Cahard, D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J. Fluor. Chem. 128, 975–996 (2007)
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010)
Morimoto, H., Tsubogo, T., Litvinas, N. D. & Hartwig, J. F. A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides. Angew. Chem. Int. Ed. 50, 3793–3798 (2011)
Wang, X., Truesdale, L. & Yu, J.-Q. Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter. J. Am. Chem. Soc. 132, 3648–3649 (2010)
Kino, T. et al. Trifluoromethylation of various aromatic compounds by CF3I in the presence of Fe(II) compound, H2O2 and dimethylsulfoxide. J. Fluor. Chem. 131, 98–105 (2010)
Qi, Q., Shen, Q. & Lu, L. Polyfluoroalkylation of 2-aminothiazoles. J. Fluor. Chem. 133, 115–119 (2012)
Kamigata, N., Ohtsuka, T., Fukushima, T., Yoshida, M. & Shimizu, T. Direct perfluoroalkylation of aromatic and heteroaromatic compounds with perfluoroalkanesulfonyl chlorides catalysed by a ruthenium(II) phosphine complex. J. Chem. Soc. Perkin Trans. I 1339–1346 (1994)
Nagib, D. A. & MacMillan, D. W. C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480, 224–228 (2011)
Fier, P. S. & Hartwig, J. F. Copper-mediated difluoromethylation of aryl and vinyl iodides. J. Am. Chem. Soc. 134, 5524–5527 (2012)
Hu, J., Zhang, W. & Wang, F. Selective difluoromethylation and monofluoromethylation reactions. Chem. Commun. 7465–7478 (2009)
Zhao, Y. & Hu, J. Palladium-catalyzed 2,2,2-trifluoroethylation of organoboronic acids and esters. Angew. Chem. Int. Ed. 51, 1033–1036 (2012)
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007)
Li, Y. et al. Stereoselective nucleophilic monofluoromethylation of N-(tert-butanesulfinyl)imines with fluoromethyl phenyl sulfone. Org. Lett. 8, 1693–1696 (2006)
Raymond, J. I. & Andrews, L. Matrix reactions of fluorohalomethanes with alkali metals. Infrared spectrum and bonding in the monofluoromethyl radical. J. Phys. Chem. 75, 3235–3242 (1971)
Russell, G. A., Guo, D. & Khanna, R. K. Alkylation of pyridine in free radical chain reactions utilizing alkylmercurials. J. Org. Chem. 50, 3423–3425 (1985)
Han, C. & Buchwald, S. L. Negishi coupling of secondary alkylzinc halides with aryl bromides and chlorides. J. Am. Chem. Soc. 131, 7532–7533 (2009)
Knop, K., Hoogenboom, R., Fischer, D. & Schubert, U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010)
Acknowledgements
We thank D.-H. Huang and L. Pasternack for NMR spectroscopic assistance, A. Schuyler and W. Uritboonthai for mass spectrometry assistance and D. Cayer for the analysis of the β-lactamase activity. Financial support for this work was provided by the US NIH/NIGMS (GM-073949), the Uehara Memorial Foundation (postdoctoral fellowship for Y.F.), the US–UK Fulbright Commission (postdoctoral fellowship for F.O.), Aarhus University, OChem Graduate School, CDNA, CFIN and NABIIT (fellowship for E.D.F.), the US NIH (graduate fellowship for R.A.R.) and Pfizer Inc. (postdoctoral fellowship for R.D.B.).
Author information
Authors and Affiliations
Contributions
Y.F., J.A.D., D.D.D., R.A.R. and P.S.B. conceived the work; Y.F., J.A.D., F.O., E.D.F., D.D.D., R.A.R., R.D.B., B.H., N.S. and M.R.C. performed the experiments; Y.F., J.A.D., F.O., E.D.F., D.D.D., R.A.R., R.D.B., B.H., N.S., M.R.C. and P.S.B. designed the experiments and analysed the data; and F.O., R.A.R., Y.I. and P.S.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This fie contains Supplementary Text and Data (see Table of Contents for more details). (PDF 16337 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Fujiwara, Y., Dixon, J., O’Hara, F. et al. Practical and innate carbon–hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012). https://doi.org/10.1038/nature11680
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11680
- Springer Nature Limited
This article is cited by
-
Predictive Minisci late stage functionalization with transfer learning
Nature Communications (2024)
-
Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres
Nature Chemistry (2023)
-
C–H Bond Functionalization of N-Heteroarenes Mediated by Selectfluor
Topics in Current Chemistry (2023)
-
Synthesis of chiral sulfinate esters by asymmetric condensation
Nature (2022)
-
Late-stage C–H functionalization offers new opportunities in drug discovery
Nature Reviews Chemistry (2021)