Abstract
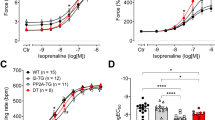
G-PROTEIN-COUPLED receptors are thought to have an inactive conformation (R), requiring an agonist-induced conformational change for receptor/G-protein coupling1–3. But new evidence suggests a two-state model4–19 in which receptors are in equilibrium between the inactive conformation (R), and a spontaneously active conformation (R*) that can couple to G protein in the absence of ligand (Fig. 1). Classic agonists have a high affinity for R* and increase the concentration of R*, whereas inverse agonists have a high affinity for R and decrease the concentration of R*. Neutral competitive antagonists have equal affinity for R and R* and do not displace the equilibrium, but can competitively antagonize the effects both of agonists and of inverse agonists. The lack of suitable in vivo model systems has restricted the evidence for the existence of inverse agonists to computer simulations7,8 and in vitro systems5,9–12,20–23. We have used a transgenic mouse model in which there is such marked myocardial overexpression of β2-adrenoceptors that a significant population of spontaneously activated receptor (R*) is present, inducing a maximal response without agonist24. We show that the β2-adrenoceptor ligand ICI-118,551 functions as an inverse agonist, providing evidence supporting the existence of inverse agonists and validating the two-state model of G-protein-coupled receptor activation.
Similar content being viewed by others
References
Clark, A. J. in The Mode of Action of Drugs on Cells (Edward Arnold, London, 1933).
Ahriens, E. J. Arch Int. Pharmacodyn. 99, 32–49 (1954).
Stephenson, R. P. Br. J. Pharmacol. 11, 379–393 (1956).
De Lean, A., Stadel, J. M. & Lefkowitz, R. J. J. biol. Chem. 255, 7108–7117 (1980).
Samama, P., Cotecchia, S., Costa, T. & Lefkowitz, R. J. J. biol. Chem. 268, 4625–4636 (1993).
Ehlert, F. J. Trends pharmacol. Sci. 7, 28–32 (1986).
Costa, T., Ogino, Y., Munson, P. J., Onaran, O. & Rodbard, D. Molec. Pharmacol. 41, 549–560 (1992).
Onaran, H. O., Costa, T. & Rodbard, D. Molec. Pharmacol. 43, 245–255 (1992).
Costa, T. & Herz, A. J. Proc. natn. Acad. Sci. U.S.A. 86, 7321–7325 (1989).
Costa, T., Lang, J., Gless, C. & Herz, A. Molec. Pharmacol. 37, 383–394 (1990).
Samama, P., Pei, G., Costa, T., Cotecchia, S. & Lefkowitz, R. J. Molec. Pharmacol. 45, 390–394 (1994).
Chidiac, P., Hebert, T. E., Valiquette, M., Dennis, M. & Bouvier, M. Molec. Pharmacol. 45, 490–499 (1994).
Lefkowitz, R. J., Cotecchia, S., Samama, P. & Costa, T. Trends pharmacol. Sci. 14, 303–307 (1993).
Schutz, W. & Freissmuth, M. Trends pharmacol. Sci. 13, 376–380 (1992).
Mewes, T., Dutz, S., Ravens, U. & Jakobs, K. H. Circulation 88, 2916–2922 (1993).
Gotze, K. & Jakobs, K. H. Eur. J. Pharmacol. 268, 151–158 (1994).
Monod, J., Wyman, J. & Changeux, J-P. J. molec. Chem. 12, 88–118 (1965).
Colquhoun, D. in Drug Receptors 149–181 (MacMillan, London, 1973).
Leff, P. Trends pharmacol. Sci. (in the press).
Cerione, R. A. et al. Biochemistry 23, 4519–4525 (1984).
Senogles, S. E. et al. J. biol. Chem. 262, 4860–4867 (1987).
Barker, E. L., Westphal, R. S., Schmidt, D. & Sanders-Bush, E. J. biol. Chem. 269, 11687–11690 (1994).
Tian, W-N., Duzic, E., Lanier, S. M. & Deth, R. C. Molec. Pharmacol. 45, 524–531 (1994).
Milano, C. A. et al. Science 264, 582–586 (1994).
Arch, J. R. S. & Kaumann, A. J. Mednl Res. Rev. 13, 663–729 (1993).
Schwartz, D. D. & Eikenburg, D. C. J. Pharmacol. exp. Ther. 244, 11–18 (1988).
Shenker, A. et al. Nature 365, 652–654 (1993).
Parma, J. et al. Nature 365, 649–651 (1993).
Seeman, P., Guan, H-C. & Van Toi, H. H. M. Nature 365, 441–445 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bond, R., Leff, P., Johnson, T. et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature 374, 272–276 (1995). https://doi.org/10.1038/374272a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/374272a0
- Springer Nature Limited
This article is cited by
-
Transcriptional adaptation of olfactory sensory neurons to GPCR identity and activity
Nature Communications (2022)
-
Atomic force microscopy-single-molecule force spectroscopy unveils GPCR cell surface architecture
Communications Biology (2022)
-
Selective Signal Capture from Multidimensional GPCR Outputs with Biased Agonists: Progress Towards Novel Drug Development
Molecular Diagnosis & Therapy (2022)
-
Trace amines produced by skin bacteria accelerate wound healing in mice
Communications Biology (2020)
-
Phenylephrine, a common cold remedy active ingredient, suppresses uterine contractions through cAMP signalling
Scientific Reports (2018)