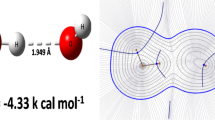
Abstract
IT is known that nitric oxide is an interesting case of a three-electron bond. Taking the x-axis along the line of centres, the isolated atoms may be described as:  The molecular orbital description of the molecule is then1:
The molecular orbital description of the molecule is then1:  The two electrons in the MO (σ2p
x) form a σ bond, the four in (π2p
y), (π2p
z)—degenerate orbitals—form π bonds, whereas the remaining single electron in (π*2p
z) is antibonding.
The two electrons in the MO (σ2p
x) form a σ bond, the four in (π2p
y), (π2p
z)—degenerate orbitals—form π bonds, whereas the remaining single electron in (π*2p
z) is antibonding.
Similar content being viewed by others
References
Coulson, C. A., “Valence”, 152 (Clarendon Press, Oxford, 1952).
Gordy, W., “Microwave Spectroscopy”, 295 (John Wiley and Sons, Inc., New York, 1953).
Greenhow, C., and Smith, W. V., J. Chem. Phys., 19, 1298 (1951).
Mulliken, R. S., et al., J. Chem. Phys., 17, 1248 (1959).
Hill, R. M., and Smith, W. V., Phys. Rev., 82, 451 (1951).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
NARASIMHA RAO, D. Molecular Quadrupole Moment of Nitric Oxide. Nature 186, 881–882 (1960). https://doi.org/10.1038/186881a0
Issue Date:
DOI: https://doi.org/10.1038/186881a0
- Springer Nature Limited