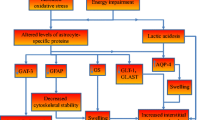
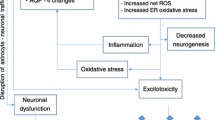
Abstract
Thiamine deficiency is a recognized cause of Wernicke's encephalopathy (WE), a condition in which small necrotic lesions are found in close proximity to the third and fourth ventricles and the Sylvian aqueduct. Although the neuropathology of WE is well-established, the pathogenic mechanisms that determine the formation and distribution of brain lesions identified with this illness are not understood. It is proposed here that glutamate neurotoxicity causes the brain lesions in WE. Glutamic acid decarboxylase (GAD), an enzyme mainly confined to the central nervous system, protects most regions of the brain from glutamate that accumulates when the activity of α-ketoglutarate dehydrogenase, a thiamine-dependent enzyme complex, is reduced. During severe thiamine deficiency, glutamate accumulates in GAD-free peripheral tissues and reaches a concentration in blood at which it passes through circumventricular organs into the cerebral ventricles or contiguous brain and finally diffuses into the extracellular space of proximate diencephalic and brain stem tissues. Extracellular glutamate eventually reaches neurotoxic levels in those tissues and causes the characteristic lesions of WE.
Similar content being viewed by others
REFERENCES
Aikawa, H., Watanabe, I.S., Furuse, T., Iwasaki, Y., Satoyoshi, E., Sumi, T. and Moroji, T. (1984). Low energy levels in thiamine-deficient encephalopathy. J. Neuropath. Exp. Neurol. 43:276–287.
Armstrong-James, M., Ross, D.T., Chen, F. and Ebner, F.F. (1988). The effect of thiamine deficiency on the structure and physiology of the rat forebrain. Metab. Brain Dis. 3:91–124.
Benveniste, H., Drejer, J., Schousboe, A. and Diemer, N.H. (1984). Elevation of the extracellular concentration of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43:1369–1374.
Bouldin, T.W. and Krigman, M.R. (1975). Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 99:444–448.
Brightman, M.W. (1965). The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am. J. Anat. 117:193–220.
Brightman, M.W. (1975). Ultrastructural characteristics of adult choroid plexus: Relation to the blood-cerebrospinal fluid barrier to proteins. In (M.G. Netsky and S. Shuangshoti, eds.) The Choroid Plexus in Health and Disease, University Press of Virginia, Charlottesville, pp. 86–112.
Brightman, M.W. and Reese, T.S. (1969). Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell. Biol. 40:648–677.
Butterworth, R.F. (1982). Neurotransmitter function in thiamine-deficiency encephalopathy. Neurochem. Int. 4:449–464.
Butterworth, R.F. (1986). Cerebral thiamine-dependent enzyme changes in experimental Wernicke's encephalopathy. Metab. Brain Dis. 1:165–175.
Butterworth, R.F. (1989). Effects of thiamine deficiency on brain metabolism: Implications for the pathogenesis of the Wernicke-Korsakoff syndrome. Alcohol Alcoholism. 24:271–279.
Butterworth, R.F., Hamel, E., Landreville, F. and Barbeau, A. (1979). Amino acid changes in thiamine-deficient encephalopathy: Some implications for the pathogenesis of Friedreich's ataxia. Can. J. Neurol. Sci. 6:217–222.
Butterworth, R.F., Giguere, J.F. and Besnard, A.M. (1986). Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. Ketoglutarate dehydrogenase. Neurochem. Res. 11:567–577.
Castel, M., Sahar, A. and Erlij, D. (1974). The movement of lanthanum across diffusion barriers in the choroid plexus of the cat. Brain Res. 67:178–184.
Erdo, S.L. (1992). Non-neuronal GABA systems: An overview. In (S.L. Erdo, ed.) GABA Outside the CNS, Springer-Verlag, Berlin, pp. 97–110.
Gaitonde, M.K., Fayein, N.A. and Johnson, A.L. (1975). Decreased metabolism in vivo of glucose into amino acids of the brain of thiamine-deficient rats after treatment with pyrithiamine. J. Neurochem. 24:1215–1223.
Gibson, G.E., Ksiezak-Reding, H., Sheu, K.F.R., Mykytyn, V. and Blass, J.P. (1984). Correlation of enzymatic, metabolic, and behavioral deficits in thiamine deficiency and its reversal. Neurochem. Res. 9:803–814.
Gomez, D.G. and Potts, D.G. (1981). The lateral, third and fourth ventricle choroid plexus of the dog: A structural and ultrastructural study. Ann. Neurol. 10:333–340.
Gross, P.M. (1991). Morphology and physiology of capillary systems in subregions of the subfornical organ and area postrema. Can. J. Physiol. Pharmacol. 69:1010–1025.
Gross, P.M. and Weindl, A. (1987). Peering through the windows of the brain. J. Cereb. Blood Flow Metab. 7:663–672.
Gross, P.M., Wall, K.M., Pang, J.J., Shaver, S.W. and Wainman, D.S. (1990). Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am. J. Physiol. 259:R1131–R1138.
Gubler, C.J., Adams, B.L., Hammond, B., Chuan-Yuan, E., Guo, S.T.I. and Bennion M. (1974). Effect of thiamin deprivation and thiamin antagonists on the level of aminobutyric acid and on 2-oxo-glutarate metabolism in rat brain. J. Neurochem. 22:831–836.
Gullotta, F. (1968). Lokalisation der Wernicke-encephalopathie im kindes-und erwachsenenalter. Arch. Psychiat. Nervenkr. 211:88–108.
Harata, N. and Iwasaki, Y. (1996). The blood-brain barrier and selective vulnerability in experimental thiamine-deficiency encephalopathy in the mouse. Metab. Brain Dis. 11:55–69.
Hazell, A.S., Butterworth, R.F. and Hakim, A.M. (1993). Cerebral vulnerability is associated with selective increase in extracellular glutamate concentration in experimental thiamine deficiency. J. Neurochem. 61:1155–1158.
Holowach, J., Kaufmann, F., Ikossi, M.G., Thomas, C. and McDougal, D.B. (1968). The effects of a thiamine antagonist, pyrithiamine, on levels of selected metabolic intermediates and activities of thiamine dependent enzymes in brain and liver. J. Neurochem. 15:621–631.
Kvamme, E., Schousboe, A., Hertz, L., Torgner, I.A. and Svenneby, G. (1985). Developmental change of endogenous glutamate and gamma-glutamyl transferase in cultured cerebral cortical interneurons and cerebellar granule cells, and in mouse cerebral cortex and cerebellum in vivo. Neurochem. Res. 10:993–1008.
Lajtha, A., Berl, S. and Waelsch, H. (1959). Amino acid and protein metabolism of the brain. J. Neurochem. 3:322–332.
Langlais, P.J. and Mair, R.G. (1990). Protective effects of the glutamate antagonist MK-801 on pyrithiamine-induced lesions and amino acid changes in rat brain. J. Neurosci. 10:1664–1674.
Langlais, P.J. and Zhang, S.X. (1993). Extracellular glutamate is increased in thalamus during thiamine deficiency and is blocked by MK-801. J. Neurochem. 61:2175–2182.
Langlais, P.J., Mair, R.G., Anderson, C.D. and McEntee, W.J. (1988). Long-lasting changes in regional brain amino acids and monoamines in recovered pyrithiamine treated rats. Neurochem. Res. 13:1199–1206.
Mair, R.G., Anderson, C.D., Langlais, P.J. and McEntee, W.J. (1988). Behavioral impairments, brain lesions and monoaminergic activity in the rat following recovery from a bout of thiamine deficiency. Behav. Brain. Res. 27:223–239.
Mancall, E.L. and McEntee, W.J. (1965). Alterations of the cerebellar cortex in nutritional encephalopathy. Neurology. 15:303–313.
McCandless, D.W. (1982). Energy metabolism in the lateral vestibular nucleus in pyrithiamine-induced thiamine deficiency. Ann. N.Y. Acad. Sci. 378:355–364.
McCandless, D.W. and Schenker, S. (1968). Encephalopathy of thiamine deficiency: Studies of intracerebral mechanisms. J. Clin. Invest. 47:2268–2280.
McCandless, D.W. and Schwartzenburg, F.C. (1981). The effect of thiamine deficiency on energy metabolism in cells of the lateral vestibular nucleus. Res. Comm. Psychol. Psychiat. Behav. 6:183–190
Novelli, A., Reilly, J.A., Lysko, P.G. and Henneberry, R.C. (1988). Glutamate becomes neurotoxic via the N-methyl-D-asparatate receptor when intracellular energy levels are reduced. Brain Res. 451:205–212.
Page, M.G., Ankoma-Sey, V., Coulson, W.F. and Bender, D.A. (1989). Brain glutamate and gamma-aminobutyrate (GABA) metabolism in thiamine-deficient rats. Brit. J. Nutr. 62:245–253.
Price, M.T., Olney, J.W., Lowry, O.H. and Bachsbaum, S. (1981). Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of the brain. J. Neurochem. 36:1774–1780.
Riggs, H.E. and Boles, R.S. (1944). Wernicke's disease: A clinical and pathologic study of 42 cases. Quart. J. Stud. Alcohol. 5:361–370.
Robinson, J,K. and Mair, R.G. (1992). MK-801 protects rats from brain lesions and behavioral impairment following pyrithiamine-induced thiamine deficiency (PTD). Behav. Neurosci. 106:623–633.
Toth, E. and Lajtha, A. (1981). Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochem. Res. 6:1309–1317.
Troncoso, J.C., Johnston, M.V., Hess, K.M. Griffin, J.W. and Price, D.L. (1981). Model of Wernicke's encephalopathy. Arch. Neurol. 38:350–354.
Victor, M. and Yakovlev, P.I. (1955). S.S. Korsakoff's psychic disorder in conjunction with peripheral neuritis. Neurology. 5:394–406.
Victor, M., Adams, R.D. and Collins, G,H. (1989). The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders due to Alcoholism and Malnutrition. 2nd ed., F.A. Davis, Philadelphia.
Watanabe, I.S. and Kanabe S. (1978). Early edematous lesion of pyrithiamine-induced acute thiamine deficient encephalopathy in the mouse. J. Neuropathol. Exp. Neurol. 37:401–413.
Weindl, A. and Joynt, R.J. (1973). Barrier properties of the subcommisural organ. Arch. Neurol. 29:16–22.
Zeevalk, G.D. and Nicklas, W.J. (1990). Chemically induced hypoglycemia and anoxia: Relationship to glutamate receptor-mediated toxicity in retina. J. Pharmacol. Exp. Ther. 253:1285–1292.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McEntee, W.J. Wernicke's Encephalopathy: an Excitotoxicity Hypothesis. Metab Brain Dis 12, 183–192 (1997). https://doi.org/10.1023/B:MEBR.0000007099.18010.72
Issue Date:
DOI: https://doi.org/10.1023/B:MEBR.0000007099.18010.72