Abstract
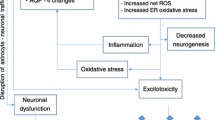
Thiamine deficiency (TD) is the underlying cause of Wernicke’s encephalopathy (WE), an acute neurological disorder characterized by structural damage to key periventricular structures in the brain. Increasing evidence suggests these focal histological lesions may be representative of a gliopathy in which astrocyte-related changes are a major feature of the disorder. These changes include a loss of the glutamate transporters GLT-1 and GLAST concomitant with elevated interstitial glutamate levels, lowered brain pH associated with increased lactate production, decreased levels of GFAP, reduction in the levels of glutamine synthetase, swelling, alterations in levels of aquaporin-4, and disruption of the blood–brain barrier. This review focusses on how these manifestations contribute to the pathophysiology of TD and possibly WE.

Similar content being viewed by others
References
Aikawa H, Watanabe IS, Furuse T, Iwasaki Y, Satoyoshi E, Sumi T, Moroji T (1984) Low energy levels in thiamine-deficient encephalopathy. J Neuropathol Exp Neurol 43:276–287
Alvarez JI, Katayama T, Prat A (2013) Glial influence on the blood brain barrier. Glia 61:1939–1958
Anzalone S, Vetreno RP, Ramos RL, Savage LM (2010) Cortical cholinergic abnormalities contribute to the amnesic state induced by pyrithiamine-induced thiamine deficiency in the rat. Eur J Neurosci 32:847–858
Armstrong-James M, Ross DT, Chen F, Ebner FF (1988) The effect of thiamine deficiency on the structure and physiology of the rat forebrain. Metab Brain Dis 3:91–124
Arriza JL, Eliasof S, Kavanaugh MP, Amara SG (1997) Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94:4155–4160
Beauchesne E, Desjardins P, Hazell AS, Butterworth RF (2009) Altered expression of tight junction proteins and matrix metalloproteinases in thiamine-deficient mouse brain. Neurochem Int 55:275–281
Beauchesne E, Desjardins P, Butterworth RF, Hazell AS (2010) Up-regulation of caveolin-1 and blood–brain barrier breakdown are attenuated by N-acetylcysteine in thiamine deficiency. Neurochem Int 57:830–837
Bender AS, Young LP, Norenberg MD (1997) Effect of lactic acid on L-glutamate uptake in cultured astrocytes: mechanistic considerations. Brain Res 750:59–66
Bergui M, Bradac GB, Zhong JJ, Barbero PA, Durelli L (2001) Diffusion-weighted MR in reversible Wernicke encephalopathy. Neuroradiol 43:969–972
Berman K, Fishman RA (1975) Thiamine phosphate metabolism and possible co-enzyme-independent functions of thiamine in brain. J Neurochem 24:457–465
Bettendorff L, Mastrogiacomo F, Kish S, Grisar T (1996) Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain. J Neurochem 66:250–258
Butterworth R (1986) Cerebral thiamine-dependent enzyme changes in experimental Wernicke’s encephalopathy. Metab Brain Dis 1:165–175
Butterworth RF, Gaudreau C, Vincelette J, Bourgault AM, Lamothe F, Nutini AM (1991) Thiamine deficiency in AIDS. Lancet 338:1086
Calingasan NY, Gibson GE (2000) Dietary restriction attenuates the neuronal loss, induction of heme oxygenase-1 and blood–brain barrier breakdown induced by impaired oxidative metabolism. Brain Res 885:62–69
Calingasan NY, Baker H, Sheu KF, Gibson GE (1994) Distribution of the alpha-ketoglutarate dehydrogenase complex in rat brain. J Comp Neurol 346:461–479
Calingasan NY, Gandy S, Baker H, Sheu K, Kim K, Wisniewski H, Gibson G (1995a) Accumulation of amyloid precursor protein-like immunoreactivity in rat brain in response to thiamine deficiency. Brain Res 677:50–60
Calingasan NY, Baker H, Sheu K, Gibson G (1995b) Blood–brain barrier abnormalities in vulnerable brain regions during thiamine deficiency. Exp Neurol 134:64–72
Calingasan NY, Park LC, Calo LL, Trifiletti RR, Gandy SE, Gibson GE (1998) Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism. Am J Pathol 153:599–610
Chan H, Butterworth RF, Hazell AS (2004) Astrocytes respond to thiamine deficiency-induced swelling by downregulating aquaporin-4 levels. Neurosci Lett 366:231–234
Collins GH (1967) Glial cell changes in the brain stem of thiamine-deficient rats. Am J Pathol 50:791–814
Combs GF Jr (2008) The vitamins: fundamental aspects in nutrition and health, 3rd edn. Elsevier Academic Press, New York
Cook C, Hallwood P, Thomson A (1998) B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol 33:317–336
Cooper JR, Roth RH, Kini MM (1963) Biochemical and physiological function of thiamine in nervous tissue. Nature 199:609–610
Cruz F, Cerdán S (1999) Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed 12:451–462
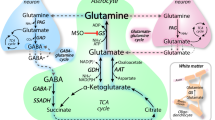
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Danbolt NC, Storm-Mathisen J, Kanner BI (1992) An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 51:295–310
Del Arco A, Segovia G, Fuxe K, Mora F (2003) Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem 85:23–33
Drejer J, Larsson OM, Schousboe A (1983) Characterization of uptake and release processes for D- and L-aspartate in primary cultures of astrocytes and cerebellar granule cells. Neurochem Res 8:231–243
Ebels EJ (1978) How common is Wernicke-Korsakoff syndrome? Lancet 2:781–782
Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG (1995) An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375:599–603
Fox JM, Duppel W (1975) The action of thiamine and its di- and triphosphates on the slow exponential decline of the ionic currents in the node of Ranvier. Brain Res 89:287–302
Gardner-Medwin AR, Coles JA, Tsacopoulos M (1981) Clearance of extracellular potassium: evidence for spatial buffering by glial cells in the retina of the drone. Brain Res 209:452–457
Gibson GE, Zhang H (2002) Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int 40:493–504
Hakim AM (1984) The induction and reversibility of cerebral acidosis in thiamine deficiency. Ann Neurol 16:673–679
Harper CG, Giles M, Finlay-Jones R (1986) Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 49:341–345
Hazell AS (2009) Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem Int 55:129–135
Hazell AS, Butterworth RF (2009) Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol 44:141–147
Hazell AS, Butterworth RF, Hakim AM (1993) Cerebral vulnerability is associated with selective increase in extracellular glutamate concentration in experimental thiamine deficiency. J Neurochem 61:1155–1158
Hazell AS, Rao KV, Danbolt NC, Pow DV, Butterworth RF (2001) Selective down-regulation of the astrocyte glutamate transporters GLT-1 and GLAST within the medial thalamus in experimental Wernicke’s encephalopathy. J Neurochem 78:560–568
Hazell AS, Pannunzio P, Rama Rao K, Pow DV, Rambaldi A (2003) Thiamine deficiency results in downregulation of the GLAST glutamate transporter in cultured astrocytes. Glia 43:175–184
Hazell AS, Sheedy D, Oanea R, Aghourian M, Sun S, Jung JY, Wang D, Wang C (2010) Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 58:148–156
Hertz L, Peng L (1992) Energy metabolism at the cellular level of the CNS. Can J Physiol Pharmacol 70:S145–S157
Holowach J, Kauffman F, Ikossi MG, Thomas C, McDougal DB Jr (1968) The effects of a thiamine antagonist, pyrithiamine, on levels of selected metabolic intermediates and on activities of thiamine-dependent enzymes in brain and liver. J Neurochem 15:621–631
Itokawa Y, Cooper JR (1970) Ion movements and thiamine. II. The release of the vitamin from membrane fragments. Biochim Biophys Acta 196:274–284
Janzer RC, Raff MC (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325:253–257
Jenkins LW, Becher DP, Coburn TH (1984) A quantitative analysis of glial swelling and ischemic neuronal injury following complete cerebral ischemia. In: Go TG, Baethmann A (eds) Recent progress in the study and therapy of brain edema. Plenum, New York, pp 523–537
Kalimo H, Rehncrona S, Söderfeldt B, Olsson Y, Siesjö BK (1981) Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab 1:313–327
Kanai Y, Hediger MA (1992) Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 360:467–471
Kimelberg HK, Rutledge E, Goderie S, Charniga C (1995) Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab 15:409–416
Kinnersley HW, Peters RA (1930) Brain localization of lactic acidosis in avitaminosis Bl and its relation to the origin of symptoms. Biochem J 24:711–722
Kokoszka JE, Coskun P, Esposito LA, Wallace DC (2001) Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A 98:2278–2283
Landau WM, Freygang WH Jr, Roland LP, Sokoloff L, Kety SS (1955) The local circulation of the living brain; values in the unanesthetized and anesthetized cat. Trans Am Neurol Assoc (80th Meeting):125–129
Langlais P, Mair R (1990) Protective effects of the glutamate antagonist MK-801 on pyrithiamine-induced lesions and amino acid changes in rat brain. J Neurosci 10:1664–1674
Langlais JP, Savage ML (1995) Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res 68:75–89
Langlais PJ, Zhang SX (1993) Extracellular glutamate is increased in thalamus during thiamine deficiency-induced lesions and is blocked by MK-801. J Neurochem 61:2175–2182
Langlais PJ, Zhang SX (1997) Cortical and subcortical white matter damage without Wernicke’s encephalopathy after recovery from thiamine deficiency in the rat. Alcohol Clin Exp Res 21:434–443
Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22:1523–1531
Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853
Lindboe CF, Loberg EM (1989) Wernicke’s encephalopathy in non-alcoholics. An autopsy study. J Neurol Sci 90:125–129
Lockman PR, Mumper RJ, Allen DD (2003) Evaluation of blood–brain barrier thiamine efflux using the in situ rat brain perfusion method. J Neurochem 86:627–634
Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M (2007) The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27:12255–12266
Mancinelli R, Ceccanti M (2009) Biomarkers in alcohol misuse: their role in the prevention and detection of thiamine deficiency. Alcohol Alcohol 44:177–182
Manz HJ, Robertson DM (1972) Vascular permeability to horseradish peroxidase in brainstem lesions of thiamine-deficient rats. Am J Pathol 66:565–576
Matsuda T, Doi T, Tonomura H, Baba A, Iwata H (1989) Postnatal development of thiamine metabolism in rat brain. J Neurochem 52:842–846
Matsushima K, MacManus JP, Hakim AM (1997) Apoptosis is restricted to the thalamus in thiamine-deficient rats. Neuroreport 8:867–870
McCandless DW (1982) Energy metabolism in the lateral vestibular nucleus in pyrithiamin- induced thiamin deficiency. Ann NY Acad Sci 378:355–364
McCandless DW, Schenker S (1968) Encephalopathy of thiamine deficiency: studies of intracerebral mechanisms. J Clin Invest 47:2268–2280
Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F (1996) GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 16:6255–6264
Miyajima Y, Fukuda M, Kojima S, Matsuyama T, Shylaja N, Aso K (1993) Wernicke’s encephalopathy in a child with acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol 15:331–334
Morishima T, Aoyama M, Iida Y, Yamamoto N, Hirate H, Arima H, Fujita Y, Sasano H, Tsuda T, Katsuya H, Asai K, Sobue K (2008) Lactic acid increases aquaporin 4 expression on the cell membrane of cultured rat astrocytes. Neurosci Res 61:18–26
Munujos P, Vendrell M, Ferrer I (1993) Proto-oncogene c-fos induction in thiamine-deficient encephalopathy. Protective effects of nicardipine on pyrithiamine-induced lesions. J Neurol Sci 118:175–180
Myers RE (1979) Lactic acid accumulation as cause of brain edema and cerebral necrosis resulting from oxygen deprivation. In: Korobkin R, Guilleminault G (eds) Advances in perinatal neurology. Spectrum, New York, pp 85–114
Nakagawasai O, Tadano T, Hozumi S, Tan-No K, Niijima F, Kisara K (2000) Immunohistochemical estimation of brain choline acetyltransferase and somatostatin related to the impairment of avoidance learning induced by thiamine deficiency. Brain Res Bull 52:189–196
Navarro D, Zwingmann C, Hazell AS, Butterworth RF (2005) Brain lactate synthesis in thiamine deficiency: a re-evaluation using 1H-13C nuclear magnetic resonance spectroscopy. J Neurosci Res 79:33–41
Nicholls D, Attwell D (1990) The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11:462–468
Ohkoshi N, Ishii A, Shoji S (1994) Wernicke’s encephalopathy induced by hyperemesis gravidarum, associated with bilateral caudate lesions on computed tomography and magnetic resonance imaging. Eur Neurol 34:177–180
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Pentschew A, Garro F (1966) Lead encephalo-myelopathy of the suckling rat and its implications on the porphyrinopathic nervous diseases. With special reference to the permeability disorders of the nervous system’s capillaries. Acta Neuropathol 6:266–278
Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI (1992) Cloning and expression of a rat brain L-glutamate transporter. Nature 360:464–467
Robertson DM, Wasan SM, Skinner DB (1968) Ultrastructural features of early brain stem lesions of thiamine-deficient rats. Am J Pathol 52:1081–1097
Roland JJ, Savage LM (2009) The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience 160:32–41
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994) Localization of neuronal and glial glutamate transporters. Neuron 13:713–725
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686
Scholz W (1949) Histologische und topische Veränderungen und Vulnerabilitätsverhältnisse im menschlichen Gehirn bei Sauerstoffmangel, Ödem und plasmatischen Infiltration. I. Problemstellung und feingewebliche Situation. Arch Psychiatr Nervenkr 181:621–665
Schousboe A (1981) Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol 22:1–45
Schousboe A, Westergaard N, Waagepetersen HS, Larsson OM, Bakken IJ, Sonnewald U (1997) Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia 21:99–105
Serres S, Raffard G, Franconi JM, Merle M (2008) Close coupling between astrocytic and neuronal metabolisms to fulfill anaplerotic and energy needs in the rat brain. J Cereb Blood Flow Metab 28:712–724
Shah N, Wolff JA (1973) Thiamine deficiency: probable Wernicke’s encephalopathy successfully treated in a child with acute lymphocytic leukemia. Pediatrics 51:750–751
Sharma A, Bist R, Bubber P (2013) Thiamine deficiency induces oxidative stress in brain mitochondria of Mus musculus. J Physiol Biochem 69:539–546
Soffer D, Zirkin H, Alkan M, Berginer VM (1989) Wernicke’s encephalopathy in acquired immune deficiency syndrome (AIDS): a case report. Clin Neuropathol 8:192–194
Sokoloff L (1981) Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab 1:7–36
Storck T, Schulte S, Hofmann K, Stoffel W (1992) Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A 89:10955–10959
Todd KG, Butterworth RF (1998) Evaluation of the role of NMDA-mediated excitotoxicity in the selective neuronal loss in experimental Wernicke encephalopathy. Exp Neurol 149:130–138
Todd KG, Butterworth RF (1999) Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia 25:190–198
Torvik A (1985) Two types of brain lesions in Wernicke’s encephalopathy. Neuropathol Appl Neurobiol 11:179–190
Tran ND, Correale J, Schreiber SS, Fisher M (1999) Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke 30:1671–1678
Tretter L, Adam-Vizi V (2000) Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci 20:8972–8979
Tretter L, Adam-Vizi V (2004) Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci 24:7771–7778
Troncoso JC, Johnston MV, Hess KM, Griffin JW, Price DL (1981) Model of Wernicke’s encephalopathy. Arch Neurol 38:350–354
Vasconcelos MM, Silva KP, Vidal G, Silva AF, Domingues RC, Berditchevsky CR (1999) Early diagnosis of pediatric. Wernicke’s encephalopathy. Pediatr Neurol 20:289–294
Victor M, Adams R, Collins G (1989) The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. F.A. Davies, Philadelphia
Vortmeyer AO, Colmant HJ (1988) Differentiation between brain lesions in experimental thiamine deficiency. Virchows Arch A Pathol Anat Histopathol 414:61–67
Walz W (1987) Swelling and potassium uptake in cultured astrocytes. Can J Physiol Pharmacol 65:1051–1057
Watanabe I (1978) Pyrithiamine-induced acute thiamine-deficient encephalopathy in the mouse. Exp Mol Pathol 28:381–394
Watanabe I, Kanabe S (1978) Early edematous lesion of pyrithiamine induced acute thiamine deficient encephalopathy in the mouse. J Neuropathol Exp Neurol 37:401–413
Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W (1994) Modulation of tight junction structure in blood–brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci 107:1347–1357
Yokote K, Miyagi K, Kuzuhara S, Yamanouchi H, Yamada H (1991) Wernicke encephalopathy: follow-up study by CT and MR. J Comput Assist Tomogr 15:835–838
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410
Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ (1995) Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J Neuropathol Exp Neurol 54:255–267
Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ (2011) Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol Aging 32:42–53
Zhao J, Sun X, Yu Z, Pan X, Gu F, Chen J, Dong W, Zhao L, Zhong C (2011) Exposure to Pyrithiamine Increases beta-Amyloid Accumulation, Tau Hyperphosphorylation, and glycogen synthase kinase-3 activity in the brain. Neurotox Res 19:575–583
Acknowledgments
The senior author (ASH) is a Visiting Professor in the Department of Neurology at Universidade Estadual de Campinas (UNICAMP), Campinas, São Paulo, Brazil. His laboratory at the University of Montreal is supported by the Canadian Institutes of Health Research. S.A. is the recipient of a doctoral scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afadlal, S., Labetoulle, R. & Hazell, A.S. Role of astrocytes in thiamine deficiency. Metab Brain Dis 29, 1061–1068 (2014). https://doi.org/10.1007/s11011-014-9571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-014-9571-y