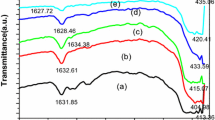
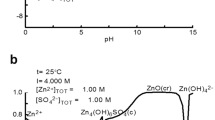
Abstract
Nanocrystalline ZnO particles were prepared from alcoholic solutions of zinc acetate dihydrate without using base such as NaOH or LiOH through a colloid process carried out at a low temperature of 60°C. A comparative study of chemical reactions from zinc acetate dihydrate to ZnO was made using different types of monool solvents, i.e. methanol, ethanol, and 2-methoxyethanol. It was revealed that layered hydroxide zinc acetate was formed as an intermediate and its transformation into ZnO was a key reaction step in any of the solutions. Reaction time necessary for the precipitation of ZnO was greatly influenced by the solvents used. Methanol was useful for the preparation of the ZnO nanoparticles, which were chemically pure in terms of cation impurities and exhibited green photoluminescence by the ultraviolet excitation.
Similar content being viewed by others
References
J.H. Fendler (ed.), Nanoparticles and Nanostructured Films (Wiley-VCH, Weinheim, 1998).
M. Moffitt, L. McMahon, V. Pessel, and A. Eisenberg, Chem. Mater. 7, 1185 (1995).
T. Trindade and P. O'Brien, Adv. Mater. 8, 161 (1996).
Y. Yang, J.M. Huang, S.Y. Liu, and J.C. Shen, J. Mater. Chem. 7, 131 (1997).
L.X. Chen, T. Rajh, Z.Y. Wang, and M.C. Thurnauer, J. Phys. Chem. B 101, 10688 (1997).
C. Pascal, J.L. Pascal, F. Favier, M.L.E. Moubtassim, and C. Payen, Chem. Mater. 11, 141 (1999).
E.A. Meulenkamp, J. Phys. Chem. B 102, 5566 (1998).
K.S. Weiβenrieder and J. Müller, Thin Solid Films 300, 30 (1997).
M. Anpo, K. Chiba, M. Tomonari, S. Coluccia, M. Che, and M.A. Fox, B. Chem. Soc. Jpn. 64, 543 (1991).
C. Lorenz, A. Emmerling, J. Fricke, T. Schmidt, M. Hilgendorff, L. Spanhel, and G. Müller, J. Non-Cryst. Solids 238, 1 (1998).
H. Rensmo, K. Keis, H. Lindström, S. Södergren, A. Solbrand, A. Hagfeldt, S.E. Lindquist, L.N. Wang, and M. Muhammed, J. Phys. Chem. B 101, 2598 (1997).
I. Bedja, P.V. Kamat, X. Hua, A.G. Lappin, and S. Hotchandani, Langmuir 13, 2398 (1997).
K. Keis, J. Lindgren, S.E. Lindquist, and A. Hagfeldt, Langmuir 16, 4688 (2000).
T. Yoshida, K. Terada, D. Schlettwein, T. Oekermann, T. Sugiura, and H. Minoura, Adv. Mater. 12, 1214 (2000).
U. Koch, A. Fojtik, H. Weller, and A. Henglein, Chem. Phys. Lett. 122, 507 (1985).
D.W. Bahnemann, C. Kormann, and M.R. Hoffmann, J. Phys. Chem. 91, 3789 (1987).
L. Spanhel and M.A. Anderson, J. Am. Chem. Soc. 113, 2826 (1991).
P.V. Kamat and B. Patrick, J. Phys. Chem. 96, 6829 (1992).
S. Monticone, R. Tufeu, and A.V. Kanaev, J. Phys. Chem. B 102, 2854 (1998).
E.A. Meulenkamp, J. Phys. Chem. B 102, 7764 (1998).
E.M. Wong, J.E. Bonevich, and P.C. Searson, J. Phys. Chem. B 102, 7770 (1998).
S. Sakohara, M. Ishida, and M.A. Anderson, J. Phys. Chem. B 102, 10169 (1998).
Y. Inubushi, R. Takami, M. Iwasaki, H. Tada, and S. Ito, J. Colliod Interf. Sci. 200, 220 (1998).
B.S. Zou, V.V. Volkov, and Z.L. Wang, Chem. Mater. 11, 3073 (1999).
M.S. Tokumoto, S.H. Pulcinelli, C.V. Santilli, and A.F. Craievich, J. Non-Cryst. Solids 247, 176 (1999).
M.S. Tokumoto, S.H. Pulcinelli, C.V. Santilli, and V. Briois, J. Phys. Chem. B 107, 568 (2003).
Z. Hu, G. Oskam, and P.C. Searson, J. Colloid Interf. Sci. 263, 454 (2003).
S.A. Al-Baldawi and T.E. Gough, Can. J. Chem. 47, 1417 (1969).
S.A. Al-Baldawi, M.H. Brooker, T.E. Gough, and D.E. Irish, Can. J. Chem. 48, 1202 (1970).
P.J. Montoya-Pelaez and R.S. Brown, Inorg. Chem. 41, 309 (2002).
T. Schmidt, G. Müller, L. Spanhel, K. Kerkel, and A. Forchel, Chem. Mater. 10, 65 (1998).
E.D. Kolb and R.A. Laudise, J. Am. Ceram. Soc. 49, 302 (1966).
B.J. Pierce and R.L. Hengehold, J. Appl. Phys. 47, 644 (1976).
R.J. Kumar, Y. Diamant, and A. Gedanken, Chem. Mater. 12, 2301 (2000).
L. Poul, S. Ammar, N. Jouini, and F. Fiévet, J. Sol-Gel Sci. Tech. 26, 261 (2003).
G.W. Tindall, LCGC North Am. 20, 1028 (2002).
L. Poul, N. Jouini, and F. Fiévet, Chem. Mater. 12, 3123 (2000).
K. Vanheusden, C.H. Seager, W.L. Warren, D.R. Tallant, and J.A. Voigt, Appl. Phys. Lett. 68, 403 (1996).
W. Stählin and H.R. Oswald, Acta Cryst. B 26, 860 (1970).
B.J. Aylett, in Comprehensive Inorganic Chemistry, edited by J.C. Bailar, H.J. Emelēus, R. Nyholm, and A.F. Trotman-Dickenson (Pergamon, Oxford, 1973), p. 223.
H.R. Oswald and R. Asper, in Preparation and Crystal Growth of Materials with Layered Structures, edited by R.M.A. Lieth (D. Reidel, Dordrecht, 1977), p. 122.
M.K. Johnson, D.B. Powell, and R.D. Cannon, Spectrochim. Acta A 38, 125 (1982).
E. Hosono, S. Fujihara, T. Kimura, and H. Imai, J. Colloid Interf. Sci. 272, 391 (2004).
A.C. Pierre, Introduction to Sol-Gel Processing (Kluwer, Boston, 1998), p. 31.
S. Ahrland, in The Chemistry of Nonaqueous Solvents, Vol. VA, edited by L.L. Lagowski (Academic Press, New York, 1978), p. 10.
S. Yamabi and H. Imai, J. Mater. Chem. 12, 3773 (2002).
Y. Inada, H. Hayashi, K. Sugimoto, and S. Funahashi, J. Phys. Chem. A 103, 1401 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hosono, E., Fujihara, S., Kimura, T. et al. Non-Basic Solution Routes to Prepare ZnO Nanoparticles. Journal of Sol-Gel Science and Technology 29, 71–79 (2004). https://doi.org/10.1023/B:JSST.0000023008.14883.1e
Issue Date:
DOI: https://doi.org/10.1023/B:JSST.0000023008.14883.1e