Abstract
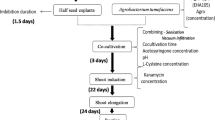
Conditions affecting Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merr.], including seed vigor of explant source, selection system, and cocultivation conditions, were investigated. A negative correlation between seed sterilization duration and seed vigor, and a positive correlation between seed vigor and regenerability of explants were observed in the study, suggesting that use of high vigor seed and minimum seed sterilization duration can further improve transformation efficiency. Selection schemes using glufosinate or bialaphos as selective agents in vitro were assessed. Glufosinate selection enhanced soybean transformation as compared to bialaphos. The use of 6 mg L-1 glufosinate during shoot induction and shoot elongation stages yielded higher final transformation efficiency ranging from 2.0% to 6.3% while bialaphos at 4 to 6 mg L-1 gave 0% to 2.1% efficiency. Including cysteine and DTT during cocultivation increased the transformation efficiency from 0.2–0.9% to 0.6–2.9%. This treatment also improved T-DNA transfer as indicated by enhanced transient GUS expression. Shoot regeneration and Agrobacterium infection were attained in twelve soybean cultivars belonging to maturity groups I-VI. These cultivars maybe amenable to genetic transformation and may provide a valuable tool in soybean improvement programs.
Similar content being viewed by others
References
An, G., P.R. Evert, A. Mitra & S.B. Ha, 1988. Binary vectors. In: S.B. Gelvin & R.A. Schilperoort (Eds.), Plant Molecular Biology Manual A3, pp. 1–19. Kluwer Academic Publishers, Dordrecht.
Baker, C.M., N. Muñoz-Fernandez & C.D. Carter, 1999. Im-proved shoot development and rooting from mature cotyledons of sunflower. Plant Cell Tiss Org Cult 58: 39–49.
Carrington, J.C. & D.D. Freed, 1990. Cap-independent enhance-ment of translation by a plant potyvirus 5' nontranslated region. J Virol 64: 1590–1597.
Clemente, T., B.J. LaValle, A.R. Howe, D.C. Ward, R.J. Rozman, P.E. Hunte, D.L. Broyles, D.S. Kasten & M.A. Hinchee, 2000. Progeny analysis of glyphosate selected transgenic soybeans de-rived from Agrobacterium-mediated transformation. Crop Sci 40: 797–803.
De Cleene, M. & J. De Ley, 1976. The host range of crown gall. Bot Rev 42: 389–466.
Delouche, J.C. & C.C. Baskin, 1973. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci & Technol 1: 427–452.
Dennehey, B.K., W.L. Peterson, C. Ford-Santino, M. Pajeau & C.L. Armstrong, 1994. Comparison of selective agents for use with the selectable marker gene bar in maize transformation. Plant Cell Tiss Org Cult 36: 1–7
Di, R., V. Purcell, G.B. Collins & S.A. Ghabrial, 1996. Production of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene. Plant Cell Rep 15: 746–750.
Donaldson, P.A. & D.H. Simmonds, 2000. Susceptibility to Agrobacterium tumefaciens and cotyledonary node transforma-tion in short-season soybean. Plant Cell Rep 19: 478–484.
Droste, A., G. Pasquali & M.H. Bodanese-Zanettini, 2002. Trans-genic fertile plants of soybean [Glycine max (L). Merrill] ob-tained from bombarded embryogenic tissue. Euphytica 127: 367–376.
Enriquez-Obregon, G.A., D.L. Prieto-Samsonov, G.A. de la Riva, M. Perez, G. Selman-Housein & R.I. Vasquez-Padron, 1999. Agrobacterium-mediated Japonica rice transformation: a proced-ure assisted by antinecrotic treatment. Plant Cell Tiss Org Cult 59: 159–168.
Frame, B.R., H. Shou, R.K. Chikwamba, Z. Zhang, C. Xiang, T.M. Fonger, S.E.K. Pegg, B. Li, D.S. Nettleton, D. Pei & K. Wang, 2002. Agrobacterium tumefaciens-mediated transforma-tion of maize embryos using standard binary vector system. Plant Physiol 129: 13–22.
Fukuoka, H., T. Ogawa, I. Mitsuhara, T. Iwai, K. Isuzigawa, Y. Nishizawa, Y. Gotoh, Y. Nishizawa, A. Tagiri & M. Ugaki, 2000. Agrobacterium-mediated transformation of monocot and dicot plants using the NCR promoter derived from soybean chlorotic mottle virus. Plant Cell Rep 19: 815–820.
Gamborg, O.L., R.A. Miller & K. Ojima, 1968. Nutrient require-ments of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158.
Hajdukiewicz, P., Z. Svab & P. Maliga, 1994. The small ver-satile pZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994.
Hansen, G. & M.S. Wright, 1999. Recent advances in the transform-ation of plants. Trends Plant Sci 4: 226–231.
Hiei, Y., S. Ohta, T. Komari & T. Kumashiro, 1994. Efficient trans-formation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282.
Hood, E.E., G.L. Helmer, R.T. Fraley & M.D. Chilton, 1986. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol 168: 1291–1301.
Ishida, Y., H. Saito, S. Ohta, Y. Hiei, T. Komari & T. Kumashiro, 1996. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14: 745–750.
ISTA, 1999. International Rules for Seed Testing. Seed Sci Technol (Supplement) 27: 1–333.
Jefferson, R.A., T.A. Kavanagh & M.W. Bevan, 1987. GUS fusion: β-glucoronidase as a selective and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907.
Komari, T. & T. Kubo, 1999. Methods of genetic transformation: Agrobacterium tumefaciens. In: I.K. Vasil (Ed.), Molecular Improvement of Cereal Crops, pp. 43–82. Kluwer Academic Publishers, Dordrecht/Boston.
Liu, W., R.S. Torisky, K.P. McAllister, S. Avdiushko, D. Hildebrand & G.B. Collins, 1996. Somatic embryo cycling: evaluation of a novel transformation and assay system for seed-specific gene expression in soybean. Plant Cell Tiss Org Cult 47: 33–42.
Lowe, K., B. Bowen, G. Hoerster, M. Ross, D. Bond, D. Pierce & B. Gordon-Kamm, 1995. Germline transformation of maize follow-ing manipulation of chimeric shoot meristems. Bio/Technology 13: 677–682.
Lu, G., A. Hepburn & J. Widholm, 1994. A simple procedure for the expression of genes in transgenic soybean callus tissue. Plant Cell Rep 13: 632–636.
Maruyama, E.K., A. Ishi, S. Migita & K. Migita, 1989. Screening of suitable sterilization of explants and proper media for tissue culture of eleven tree species of Peru-Amazon forest. J Agric Sci 33: 252–261.
Mason, H.S., D. DeWald & J.E. Mullet, 1993. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell 5: 241–251.
Maughan, P.J., R. Phili, M.-J. Cho, J.M. Widholm & L.O. Vodkin, 1999. Biolistic transformation, expression, and inheritance of bovine β-casein in soybean (Glycine max). In Vitro Cell Dev Biol-Plant 35: 334–349.
Meurer, C.A., R.D. Dinkins & G.B. Collins, 1998. Factors affecting soybean cotyledonary node transformation. Plant Cell Rep 18: 180–186.
Murashige, T. & F. Skoog, 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–479.
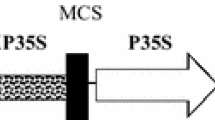
Odell, J.T., F. Nagy & N.H. Chua, 1985. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 6: 810–812.
Olhoft, P.M., K. Lin, J. Galbraith, N.C. Nielsen & D.A. Somers, 2001. The role of thiol compounds in increasing Agrobacterium-mediated transformation of soybean cotyledonary-node cells. Plant Cell Rep 20: 731–737.
Olhoft, P.M. & D.A. Somers, 2001. L-cysteine increases Agrobacte-rium-mediated T-DNA delivery into soybean cotyledoanry-node cells. Plant Cell Rep 20: 706–711.
Olhoft, P.M., L.E. Flagel, C.M. Donovan & D.A. Somers, 2003. Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216: 723–735.
Parrott, W.A., L.M. Hoffman, D.F. Hildebrand, E.G. Williams & G.B. Collins, 1989. Recovery of primary transformants of soybean. Plant Cell Rep 7: 615–617.
Peach, C. & J. Velten, 1992. Application of the chloramphen-icol acetyltransferase (CAT) diffusion assay to transgenic plant tissues. BioTechniques 12: 181–186.
Perl, A., O. Lotan, M. Abu-Abied & D. Holland, 1996. Estab-lishment of an Agrobacterium-mediated transformation system for grape (Vitis vinifera L.): The role of antioxidants during grape-Agrobacterium interactions. Nat Biotechnol 14: 624–628.
Reddy, M.S.S., R.D. Dinkins & G.B. Collins, 2003. Gene si-lencing in transgenic soybean plants transformed via particle bombardment. Plant Cell Rep 21: 676–683.
Sandhu, D., J.A. Champoux, S.N. Bondareva & K.S. Gill, 2001. Identification and physical localization of useful genes and mark-ers to a major gene-rich region on wheat group 1S chromosomes. Genetics 157: 1735–1747.
Santarem, E.R. & J.J. Finer, 1999. Transformation of soybean [Gly-cine max (L.) Merrill] using proliferative embryogenic tissue maintained on semi-solid medium. In Vitro Cell Dev Biol – Plant 35: 451–455.
Santarem, E.R., H.N. Trick, J.S. Essig & J.J. Finer, 1998. Sonication-assisted Agrobacterium-mediated transformation of soybean immature cotyledons: optimization of transient expres-sion. Plant Cell Rep 17: 752–759.
Singh, R.J., T.M. Klein, C.J. Mauvais, S. Knowlton, T. Hymowitz & C.M. Kostow, 1998. Cytological characterization of transgenic soybean. Theor Appl Genet 96: 319–324
Shou, H., B. Frame, S. Whitham & K. Wang, 2004. Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol Breed, in press, http: //www.kluweronline.com/issn/1380-3743/contents
Somers, D.A., D.A. Samac & P.M. Olhoft, 2003. Recent advances in legume transformation. Plant Physiol 131: 892–899.
Tachibana, K., T. Watanabe, Y. Sekizawa & T. Takematsu, 1986. Accumulation of ammonia in plants treated with bialaphos. J Pestic Sci 11: 33–37.
Thompson, C.K., N.R. Movva, R. Tizard, R. Crameri, J.E. Davies, M. Lauwereys & J. Botterman, 1987. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6: 2519–2523.
Trick, H.N. & J.J. Finer, 1998. Sonication-assisted Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merril] embryogenic suspension culture tissue. Plant Cell Rep 17: 482–488.
Vancanneyt, G., R. Schmidt, A. O'Connor-Sanchez, L. Willmitzer & M. Rocha-Sosa, 1990. Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220: 245–250.
Wang, K., B. Frame & L. Marcell, 2003. Genetic transformation of maize. In: P.K. Jaiwal & R.P. Singh (Eds.), Plant Engineer-ing Vol. 2, Improvement of Food Crops, pp. 175–217. Sci Tech Publishing LLC, USA.
Wehrmann, A., A.V. Vliet, C. Opsomer, J. Botterman & A. Schulz, 1996. The similarities of bar and pat gene products make them equally applicable for plant engineers. Nat Biotechnol 14: 1274–1278.
White, J., S.Y. Chang, M.J, Bibb & M.J. Bibb, 1990. A cassette containing the bar gene of S. hygroscopicus: a selectable marker for plant transformation. Nucl Acids Res 18: 1062.
Widholm, J.M., 1995. Leguminous plants. In: K. Wang, A. Herrera-Estrella & M. Van Montagu (Eds.), Transformation of Plants and Soil Microorganisms, pp. 101–124. Cambridge University Press, Cambridge.
Zhang, Z., A. Xing, P. Staswick, T. Clemente, 1999. The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tiss Org Cult 56: 37–46.
Zhang, Z., Z. Guo, H. Shou, S.E. Pegg, T.E. Clemente, P.E. Staswick & K. Wang, 2000. Assessment of conditions affecting Agrobacterium-mediated soybean transformation and routine re-covery of transgenic soybean. In: A.D. Arencibia (Ed.), Plant Genetic Engineering: Towards the Third Millennium: Proceed-ings of the International Symposium on Plant Genetic Engineer-ing 6–10 December 1999, Havana, Cuba, pp. 88–94. Elsevier, Amsterdam / New York.
Zhao, Z.Y., W. Gu, C. Tishu, L. Tagliani, D. Hondred, D. Bond, S. Schroeder, M. Rudert & D. Pierce, 2001. High throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol Breed 8: 323–333.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paz, M.M., Shou, H., Guo, Z. et al. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136, 167–179 (2004). https://doi.org/10.1023/B:EUPH.0000030669.75809.dc
Issue Date:
DOI: https://doi.org/10.1023/B:EUPH.0000030669.75809.dc