Abstract
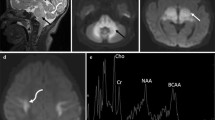
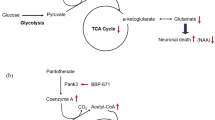
Summary: Nuclear magnetic resonance (NMR) spectroscopy is a safe, noninvasive method that is the preferred technique for in vivo analysis of specific chemical compounds in localized brain regions. Besides quantification of compounds, NMR spectroscopy allows the detailed analysis of neurotransmitter, glucose and lactate metabolism following peripheral infusions of stable isotopically labelled precursors. The latter has been successfully applied to patients with different neurological disease states not including glutaryl-CoA dehydrogenase (GCDH) deficiency. In contrast, single patients with GCDH deficiency who were neurologically unremarkable have been studied with conflicting results. One patient was shown to have an increase in intracerebral creatine and phosphocreatine concentrations, while the second studied had unremarkable levels. In a 15-year-old patient, we were able to demonstrate elevated levels of intracerebral lactate and elevated choline/N-acetylaspartate ratios, indicating potentially increased myelin turnover and reduced neuronal integrity in periventricular white matter. Interestingly, spectra in basal ganglia were within normal limits. Systematic studies to address well-defined questions in GCDH deficiency are urgently needed. In particular, analysis of in vivo neurotransmitter metabolism following administration of isotopically labelled precursors in patients with GCDH deficiency, both when metabolically stable and when unstable, may help to advance our understanding of the pathophysiology of GCDH deficiency.
Similar content being viewed by others
REFERENCES
Bähr O, Mader I, Zschocke J, et al (2002)Adult onset glutaric aicduria type I presenting with aleukoencephalopathy.Neurology 59:1802-1804.
Gruber S, Frey R, Mlynarik V, et al (2003a)Quanti fication of metabolic diüerences in the frontal brain of depressive patients and controls obtained by 1 H-MRS at 3 Tesla.Invest Radiol 38:403-408.
Gruber S, Mlynarik V, Moser E (2003b)High-resolution 3D proton spectroscopic imaging of the human brain at 3T:SNR issues and application for anatomy-matched voxel sizes.Magn Reson Med 49:299-306.
Gruetter R, Novotny EJ, Boulware SD, et al (1994)Localized 13 C NMR spectroscopy in the human brain of amino acid labeling from D-(1-13 C)glucose.J Neurochem 63:1377-1385.
Gruetter R, Adriany G, In-Young C, et al (2003)Localized in vivo 13 C NMR spectroscopy of the brain.NMR Biomed 16:313-338.
Hammen T, Stefan H, Eberhardt KE, et al (2003)Clinical applications of 1 H-MR spectroscopy in the evaluation of epilepsies What do pathological spectra stand for with regard to current results and what answers do they give to common clinical questions concerning the treatment of epilepsies?Acta Neurol Scand 108:223-238.
Mason GF, Gruetter R, Rothman DL, et al (1995)Simultaneous determination of the rates of the TCA cycle,glucose utilization,alpha-ketoglutarate/glutamate exchange and glutamine synthesis in human brain by NMR.J Cereb Blood Flow Metab 15:12-25.
Möller HE, Koch HG, Weglage J, et al (2003)Investigation of the cerebral energy status in patients with glutaric aciduria type I by 31 P magnetic resonance spectroscopy.Neuropediatrics 34:57-60.
Novotny EJ, Ashwal S, Shevell M(1998)Proton magnetic resonance spectroscopy:an emerging technology in pediatric neurology research.Pediatr Res 44:1-10.
Novotny EJ, Fulbright RK, Pearl PL, et al (2003)Magnetic resonance spectroscopy of neuro-transmitters in human brain.Ann Neurol 54-(supplement 6):S25-S31.
Pan JW, Lane JB, Hetherington H, et al (1999)Rett syndrome:1 H spectroscopic imaging at 4.1 Tesla.J Child Neurol 14:524-528.
Pouwels PJ, Brockmann K, Kruse B, e al (1999)Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res 46:474-485.
Weglage J, Wiedermann D, Denecke J, et al (2002)Individual blood-brain barrier phenylalanine transport in siblings with classical phenylketonuria.J Inherit Metab Dis 25:431-436.
Theberge J, Al-Semaan Y, Williamson PC, et al (2003)Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS.Am J sychiatry 160:2231-2233.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodamer, O.A., Gruber, S. & StÖckler-Ipsiroglu, S. Nuclear magnetic resonance spectroscopy in glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis 27, 877–883 (2004). https://doi.org/10.1023/B:BOLI.0000045772.09776.e0
Issue Date:
DOI: https://doi.org/10.1023/B:BOLI.0000045772.09776.e0