Abstract
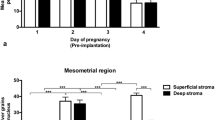
A 32 kDa estrogen-induced, sialic acid-specific agglutinin (P-SAS) was isolated from rat endometrium in its proestrus stage [1]. To investigate the functional importance of P-SAS in the uterine milieu, specific binding assays were carried out with 125I-labeled P-SAS and different cellular components of the uterus (epithelial, stromal and myometrial cells), that were isolated from different stages of the estrus cycle. The results indicate that although the protein is secreted from the epithelial cells in the estrogenic phase, it binds specifically to the stromal cells, especially to those isolated from the diestrus stage of the estrus cycle. The specific binding, however, is seen to decrease with the progression of pregnancy. Scatchard analysis performed with varying amounts of 125I-P-SAS in the presence of excess cold P-SAS revealed that the binding occurs with a Ka = 1.69 × 108 M-1. As P-SAS binds specifically to sialic acids on the stromal cell surface, further characterization of the sialic acid molecule to which P-SAS binds was carried out by gas liquid chromatography (GLC). The studies revealed that P-SAS preferentially binds to N-glycolylneuraminic acid, which is attached to the penultimate sugar of the stromal cell surface glycoprotein chain via α2,6 linkage. As P-SAS is further known to be mitogenic [2], the effect of P-SAS on cultured stromal cells was studied in vitro. The growth regulatory assays revealed that P-SAS induced 3H-thymidine uptake by stromal cells in culture. Thus, from the above observations, paracrine effects of P-SAS on the stromal cells and on the subsequent growth and development of the uterus can be assumed.
Similar content being viewed by others
References
Chowdhury M, Sarkar M, Mandal C: Identification and isolation of an agglutinin from uterus of rats. Biochem Biophys Res Commun 130: 1301–1307, 1985
Bhattacharyya I, Mandal C, Chowdhury M: Functional heterogeneity of sialic acid binding agglutinins of rat uteri towards in vitro lymphocyte transformation. Am J Reprod Immunol 20: 81–86, 1989
Ivatt RJ: Role of glycoproteins during early mammalian embryogenesis. In: The Biology of Glycoproteins. Plenum Press, New York, 1984, pp 95–181
Boströ m H, Odelblad E: Autoradiographic observations on the uptake of S35 in the genital organs of the female rat and rabbit after injection of labelled sodium sulfate. Acta Endocrinol Copenhagen 10: 89–96, 1952
Walter I, Bavdek S: Lectin binding patterns of porcine oviduct mucosa and endometrium during the oestrous cycle. J Anat 190: 299–307, 1997
Dutt A, Tang JP, Carson DD: Estrogen preferentially stimulates lactosaminoglycan-containing oligosaccharide synthesis in mouse uteri. J Biol Chem 263: 2270–2279, 1988
Dutt A, Tang JP, Welply JK, Carson DD: Regulation of N-linked glycoprotein assembly in uteri by steroid hormones. Endocrinology 118: 661–673, 1986
Varki A: Sialic acids as ligands in recognition phenomena. FASEB J 11: 248–255, 1997
Kelm S, Schauer R: Sialic acids in molecular and cellular interactions. Int Rev Cytolog 175: 137–240, 1997
Jones CJP, Kimber SJ, Illingworth I, Aplin JD: Decidual sialylation showed species-specific differences in the pregnant mouse and rat. J Reprod Fertil 106: 241–250, 1996
Weitlauf HM: The biology of implantation. In: E. Knobil, J.D. Neill (eds). The Physiology of Reproduction. Raven Press, New York, 1994, pp 391–440
Tang B, Guller S, Gurpide E: Mechanism of human endometrial stromal cell decidualization. Ann NY Acad Sci 734: 19–25, 1994
Kimber SJ, Illingworth I, Glasser SR: Expression of carbohydrate antigens in the rat uterus during early pregnancy and after ovariectomy and steroid replacement. J Reprod Fertil 103: 75–87, 1995
Jones CJP, Aplin JD, Mulholland J, Glasser SR: Patterns of sialylation in differentiating rat decidual cells as revealed by lectin histochemistry. J Reprod Fertil 99: 635–645, 1993
Zhu ZM, Kojima N, Stroud MR, Hakomori SI, Fenderson BA: Monoclonal antibody directed to Ley oligosaccharide inhibits implantation in the mouse. Biol Reprod 52: 903–912, 1995
Nelson JD, Jato-Rodriguez JJ, Mookerjea S: Effect of ovarian hormones on glycosyltransferase activities in the endometrium of ovariectomized rats. Arch Biochem Biophys 169: 181–191, 1975
Banerjee M, Chowdhury M: Purification and characterization of a sperm-binding glycoprotein from human endometrium. Human Reprod 9: 1497–1504, 1994
Chakraborty I, Chowdhury M: Activation of epididymal sperm by sialic acid-binding (SAS) agglutinin of uterus in rats. Mol Androl 5: 59–68, 1993
Banerjee M, Chowdhury M: Induction of capacitation in human spermatozoa in vitro by an endometrial sialic acid-binding lectin. Human Reprod 10: 3147–3153, 1995
Chakraborty I, Mandal C, Chowdhury M: Modulation of sialic acidbinding proteins of rat uterus in response to changing hormonal milieu. Mol Cell Biochem 126: 7–86, 1993
Kohn J, Wilchek M: A new approach (cyanotransfer) for cyanogen bromide activation of sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun 107: 878–884, 1982
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951
McCormack SA, Glasser SR: Differential response of individual cell types from immature rats treated with estradiol. Endocrinol 106: 1634–1649, 1980
Satyaswaroop PG, Bressler RS, de la Pena MM, Gurpide E: Isolation and culture of human endometrial glands. J Clin Endocrinol Metab 48: 639–641, 1979
Rajabi MR, Singh A: Cell origin and paracrine control of intestinal collagenase in the Guinea pig uterine cervix–Evidence for a low molecular weight epithelial cell-derived collagenase stimulator Biol Reprod 52: 516–523, 1995
Scatchard G: The attraction of proteins for small molecules and ions. Ann NY Acad Sci 51: 650–672, 1949
Stockert RJ, Morell AG, Scheinberg IH: Mammalian hepatic lectin. Science 186: 365–366, 1974
Veh RW, Michelski J-C, Corfield AP, Sander M, Gies D, Schauer R: A new chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides. J Chromatogr 212: 313–322, 1981
Martensson E, Raal A, Svennerholm L: Sialic acids in blood serum. Biochim Biophys Acta 30:124–129, 1958
Aminoff D: Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J 81: 384–392, 1961
Warren L: The thiobarbituric acid assay of sialic acids. J Biol Chem 234: 1971–1975, 1959
Sloneker JH: Gas liquid chromatography of alditol acetates. In: R.L. Whistler, J.N. BeMiller (eds). Methods of Carbohydrate Chemistry, vol. 6. Academic Press, New York and London, 1972, pp 20–24
Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK: Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil 101: 421–426, 1994
Watnick AS, Russo RA: Survival of skin homografts in uteri of pregnant and progesterone-estrogen treated rats. Proc Soc Exp Biol Med 128: 1–4, 1968
Roberts GP: Inhibition of lymphocyte stimulation by bovine uterine proteins. J Reprod Fertil 50: 337–339, 1977
Sherblom AP, Smagula RM, Moody CE, Anderson GW: Immunosuppresion, sialic acid, and sialyl transferase of bovine serum as a function of progesterone concentration. J Reprod Fertil 74: 509–517, 1985
Shaw L, Schauer R: The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol Chem Hoppe-Seyler 369: 477–486, 1988
Schauer R, Stoll S, Reuter G: Differences in the amount of N-acetyland N-glycoloylneuraminic acid, as well as O-acylated sialic acids, of fetal and adult bovine tissues. Carb Res 213: 353–359, 1991
Traving C, Schauer R: Structure, function and metabolism of sialic acids. Cell Mol Life Sci 54: 1330–1349, 1998
Schauer R, Malykh YN, Krisch B, Gollub M, Shaw L: Biosynthesis and biology of N-glycolylneuraminic acid. In: Y. Inoue, Y.C. Lee, F.A. Troy II (eds). Sialobiology and Other Novel Forms of Glycosylation. Gakushin Publishing Co., Osaka, 1999, pp 17–27
Muchmore EA: Developmental sialic acid modifications in rat organs. Glycobiol 2: 337–343, 1992
Bouhours J-F, Bouhours D: Hydroxylation of CMP-Neu5Ac controls the expression of N-glycolylneuraminic acid in GM3 ganglioside of the small intenstine of inbred rats. J Biol Chem 264: 16992–16999, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chatterji, U., Sen, A., Schauer, R. et al. Paracrine effects of a uterine agglutinin are mediated via the sialic acids present in the rat uterine endometrium. Mol Cell Biochem 215, 47–55 (2000). https://doi.org/10.1023/A:1026582715752
Issue Date:
DOI: https://doi.org/10.1023/A:1026582715752