Abstract
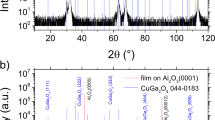
β-BaB2O4 (β-BBO) thin films were successfully synthesized by the sol-gel method using metallo-organic compounds. A stable BBO precursor solution was prepared from barium metal and boron triethoxide or 2,4,6-triethoxycyclotriboroxane in a mixture solvent of ethanol and 2-ethoxyethanol. As-precipitated powder formed by hydrolysis of the precursor solutions crystallized to γ phase, which was transformed to β phase at higher temperatures. The transformation temperatures of powders from γ to β phase of the ethoxide system and the boroxane system were 600 and 680°C, respectively. The calcination of precursor films in a mixture gas of water and oxygen was found to decrease the crystallization temperature ofβ-BBO films on Pt sheet substrates. The precursor films prepared from the ethoxide system and the boroxane system crystallized to β-BBO on Pt (111)/glass substrates at 500 and 550°C, respectively. The BBO films on Pt(111)/glass substrates showed the strong (006) preferred orientation. Theβ-BBO films on Pt(111)/glass substrates showed the second harmonic generation (SHG) of the 532 nm light on irradiation with 1064 nm light. The SH power from the BBO films was correlated with the fundamental power through the square-law proportionality based on the theory. The SHG efficiency of the BBO films was dependent upon the film thickness.
Similar content being viewed by others
References
S. Lu, M. Ho, and J. Jiang, Acta. Phys. Sinica. 31, 948 (1982).
J. Liebertz and S. Stähr, Z. Kristallogr. 165, 91 (1983).
R. Frönlich, Z. Kristallogr. 168, 109 (1984).
A.D. Mighell, A. Perloff, and S. Block, Acta. Cryst. 20, 819 (1969).
O. Yamaguchi, K. Tominaga, and K. Shimizu, Ceramurgia Intl. 6, 103 (1980).
JCPDS Cards, 35-181.
C.T. Chen, B.C. Wu, A.D. Jiang, and G.M. You, Sci. Sin. Ser. B (Eng. Ed.) 28, 235 (1985).
D. Eimerl, L. Davis, S. Velsko, E.K. Graham, and A. Zalkin, J. Appl. Phys. 62, 1968 (1987).
L.K. Cheng, W. Bosenberg, and V.L. Tang, J. Cryst. Growth 89, 553 (1988).
R.S. Feigelson, R.J. Raymeker, and R.K. Route, J. Cryst. Growth 97, 352 (1989).
K. Itoh, F. Marumo, and Y. Kuwano, J. Cryst. Growth 106, 728 (1990).
S. Hirano and K. Kato, Adv. Ceram. Mater. 2, 142 (1987).
S. Hirano and K. Kato, Adv. Ceram. Mater. 3, 503 (1988).
S. Hirano and K. Kato, J. Non-Cryst. Solid 100, 538 (1988).
S. Hirano, K. Kikuta, and K. Kato, Mat. Res. Soc. Symp. Proc. 200, 3 (1990).
S. Hirano, T. Yogo, K. Kikuta, Y. Araki, M. Saito, and S. Ogasahara, J. Amer. Ceram. Soc. 75, 2785 (1992).
S. Hirano, T. Yogo, K. Kikuta, T. Morishita, and Y. Ito, J. Amer. Ceram. Soc. 75, 1701 (1992).
S. Hirano, T. Yogo, K. Kikuta, K. Kato, W. Sakamoto, and S. Ogasahara, Ceram. Trans., Ferroelectric Films, edited by A.S. Bhalla and K.M. Nair (Amer. Ceram. Soc., Westerville, OH, 1992), vol. 25, pp. 19-32.
S. Hirano, T. Yogo, K. Kikuta, and K. Yamagiwa, J. Amer Ceram. Soc. 75, 2590 (1992).
S. Hirano, T. Yogo, K. Kikuta, K. Yamagiwa, and K. Niwa, J. Non-Cryst. Solid. 178, 293 (1994).
M.F. Lappert, J. Chem Soc. 2790 (1958).
J. Pouchert and J. Behnke, The Aldrich Library of 13 C and 1 H FT-NMR Spectra (Aldrich Chemical, Milwaukee, WI, 1992).
E. Breitmaier and W. Voelter, Carbon-13 NMR Spectroscopy (VCH, New York, 1987).
C.E. Weir and R.A. Schroeder, J. Res. Natl. Bur. Stand. 68A, 465 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yogo, T., Kikuta, K., Niwa, K. et al. Processing of β-BaB2O4 Thin Films Through Metal Organics. Journal of Sol-Gel Science and Technology 9, 201–209 (1997). https://doi.org/10.1023/A:1026425516563
Issue Date:
DOI: https://doi.org/10.1023/A:1026425516563