Abstract
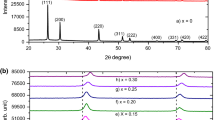
In this work, a facile chemical bath deposition process was used to prepare nanorods, nanosheets, and cauliflower PbO thin films from various cation molar concentrations. After being prepared at different molar concentrations, the as-deposited PbO thin films were annealed in a furnace at 400 °C. Subsequently, the PbO thin films were characterized using a variety of techniques. The XRD result demonstrates the presence of the β-PbO and α-PbO phases, with the β-PbO phase predominating. The average crystallite size of PbO thin film deposited at 0.1 M, 0.15 M, 0.2 M, and 0.25 M molar concentrations was 36.51 nm, 41.22 nm, 37.28 nm, and 40.38 nm, respectively. The UV–Vis spectroscopy result indicated that the optical bandgap of PbO thin films increased from 3.14 to 3. 81 eV as the lead molar concentrations varied from 0.1 to 0.25 M. The strong green emission band at the 523 nm region was shown by the PL spectroscopy. The coexistence of hexagonal nanorods, cauliflower, and networked nanosheet structures coated on the substrate’s surface is confirmed by the SEM surface morphology analysis. The existence of both Pb and O elements is confirmed by EDX analysis.








Similar content being viewed by others
References
M Suganya Sci. Pol. 32 448 (2014)
V N Suryawanshi, A S Varpe and M D Deshpande Thin Solid Films 645 87 (2018)
A O Mousa and A F Marmoss Int. J. ChemTech Res. 10 625 (2017)
C G Poll and D J Payne Electrochim. Acta 156 283 (2014)
K Konstantinov, S H Ng, J Z Wang and G X Wang J. Power Sources 159 241 (2006)
R Bapitha and M Alagar J. Adv. Chem. Sci. 3 499 (2017)
A Güngör, R Genç and T Özdemir J. Turkish Chem. Soc. Sect. A Chem. 4 1017 (2017)
N Mythili and K T Arulmozhi Int. J. Sci. Eng. Res. 5 412 (2014)
F T Geldasa, M Kebede, M W Shura and D M Andoshe Scr 98 065701 (2023)
M H Eisa Mater. Sci. Semicond. Process. 110 104966 (2020)
M Suganya, V Narasimman, J Srivind, V S Nagarethinam, A K Usharani and A R Balu J. Electr. Devices 21 1842 (2015)
J P Samberg Res. Bull. 51 356 (2014)
F T Geldasa, M A Kebede, M W Shura and F G Hone AIP Adv. 12 115302 (2022)
P Veluchamy and H Minoura Appl. Phys. Lett. 65 2431 (1994)
J C Schottmiller J. Appl. Phys. 37 3505 (1966)
M Salavati-Niasari F Mohandes and F Davar Polyhedron 28 2263 (2009)
F T Geldasa and M A Kebede Phys. J. Plus 138 165 (2023)
A B Gite Sci. Res. India 15 134 (2018)
N Yu and L Gao Acta 54 3835 (2009)
A B Velichenko, E A Baranova, D V Girenko and R Amadelli Oper. Res. 97 131 (2000)
R Naeem, R Yahya and A Pandikumar J. Mater. Sci. Mater. Electron. 28 868 (2017)
J Harjuoja and A Kosola M Putkonen and L Niinisto Thin Solid Films 496 346 (2006)
L M Droessler Lett. 71 51 (2012)
G Hodes Phys. Chem. Chem. Phys. 9 2181 (2007)
A Ashok, G Regmi, A Romero-Núñez and M Solis-López J. Mater. Sci. Mater. Electron. 31 7499 (2020)
F G Hone and F B Dejene Mater. Res. Express 5 026409 (2018)
S K Shaikh and S I Inamdar J. Alloys Compd. 664 242 (2016)
A C Nwanya, P E Ugwuoke and B A Ezekoye Mater. Sci. Eng. 2013 2 (2013)
M I Pintor-Monroy, M Cota-Leal, D Cabrera-German and A Garzon-Fontecha Sci. Semicond. Process 72 37 (2017)
D D Eya Open Internet J 17 10 (2006)
M O Nwodo, S C Ezugwu and F I Ezema J. Chem. 3 95 (2011)
K Momma and F Izumi J. Appl. Crystallogr 44 1272 (2011)
R J Hill Acta Crystallogr. 41 1281 (1985)
C J Humphreys Acta Cryst. 69 45 (2012)
K P S Ramaswamy and M Shkir J. Mater. Sci. Mater. Electron. 31 1817 (2019)
M Shkir, I M Ashraf, S Alfaify and A M El-toni Int. 46 4652 (2019)
F G Hone and F B Dejene Inorg. Chem. Commun. 111 107583 (2019)
H D Jang and J Jeong Aerosol Sci. Technol. 23 553 (1995)
Y Wang, R Gao, H Zhao, J Li and R Zhang J. Am. Ceram. Soc. 106 1050 (2023)
M Cheraghizade Phys. A Mater. Sci. Process. 123 9 (2017)
Acknowledgements
Dr. Fekadu Gashaw acknowledges Addis Ababa University for funding his thematic research project (Grant Ref. LT/PY-242/2021). Dr. Mesfin Kebede also thanks the National Research Foundation for their support, which was provided under grant number 129269.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Geldasa, F.T., Kebede, M.A., Werta, S.Z. et al. Chemical bath deposition approach to produce three different morphologies of PbO thin films from different cation concentrations. Indian J Phys (2024). https://doi.org/10.1007/s12648-024-03083-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12648-024-03083-w