Abstract
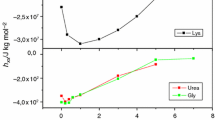
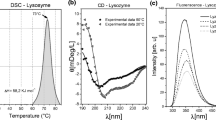
The isolation of S-, S1-, and S2-ovalbumin from domestic hen egg R-ovalbumin and of two methods for S-ovalbumin A1 are described. The first is by heat treatment of R-ovalbumin A1 and the second is of R-ovalbumin followed by fractionation on Sepharose. A kinetics and equilibrium study is made of their behavior in the presence of urea and compared with that of R-ovalbumins. As anticipated, the S-ovalbumins are much more resistant to urea than R-ovalbumins. Unlike the latter, S-ovalbumins' equilibrium profiles have a simpler sigmoidal shape. The unfolding of S1- and S2-ovalbumin is an order of magnitude slower than that of R-ovalbumin. Some possible structural differences between R- and S-ovalbumin forms and their significance are discussed.
Similar content being viewed by others
REFERENCES
Burley, R. W., and Vadehra, D. V. (1989). The Avian Egg: Chemistry and Biology, Wiley, New York, pp. 65–86.
Cho, Kon Ho (1969). The Conformation and Denaturation of Ovalbumin, Dissertation, Princeton University, Princeton, NJ.
Donovan, J. W., and Mapes, C. J. (1976). J. Sci. Food Agric. 27: 197–204.
Glazer, A. N., McKenzie, H. A., and Wake, R. G. (1963). Biochim. Biophys. Acta 69: 240–249.
Goux, W. J., and Venkatasubramanian, P. N. (1986). Biochemistry 25: 84–94.
Halliday, J. A., Bell, K., McAndrew, K., and Shaw, D. C. (1993). Protein Seq. Data Anal. 5: 201–205.
Huntington, J. A., and Stein, P. E. (2001). J. Chromatogr. B 756: 189–198.
Huntington, J. A., Patson, P. A., and Gettins, P. G. W. (1995). J. Protein Sci. 4: 613–621.
Johnson, W. C., Jr. (1990). Proteins 7: 205–214.
Kato, A., Tanaka, A., Matsudomi, N., and Kobayashi, K. (1986). Agric. Biol. Chem. 50: 2375–2376.
Kim, P. S., and Baldwin, R. L. (1990). Annu. Rev. Biochem. 59: 631–660.
Kint, S., and Tomimatsu, Y. (1979). Biopolymers 18: 1073–1079.
McKenzie, H. A. (2003). J. Protein Chem. 22: (this issue).Author please supply missing information
McKenzie, H. A., and Frier, R. D. (2003). J. Protein Chem. 22: (this issue).Author please supply missing information
McKenzie, H. A., and Shaw, D. C. (1973). Proc. Aust. Biochem. Soc. 6: 7.
McKenzie, H. A., Smith, M. B., and Wake, R. B. (1963). Biochim. Biophys. Acta 69: 222–239.
Nakamura, R., Takemori, Y., and Shitamori, S. (1981). Agric. Biol. Chem. 45: 1653–1659.
Paolinelli, C., Barteri, M., Boffi, F., Forastieri, M., Gaudiano, C., Della Longa, S., et al. (1997). Z. Naturforsch. 52: 645–653.
Simpson, R. B., and Kauzmann, W. (1953). J. Am. Chem. Soc. 75: 5139–5152.
Smith, M. B. (1964). Aust. J. Biol. Sci. 17: 264–270.
Smith, M. B., and Back, J. F. (1962). Nature 193: 878–879.
Smith, M. B., and Back, J. F. (1965). Aust. J. Biol. Sci. 18: 365–377.
Stein, P. E., Leslie, A. G. W., Finch, J. T., and Carrell, R. W. (1991). J. Mol. Biol. 221: 941–959.
Tatsumi, E., and Hirose, M. (1997). J. Biochem. 122: 300–308.
Webster, D. M., and Thompson, E. O. P. (1980). Aust. J. Biol. Sci. 33: 269–278.
Woody, R. W. (1996). In: Circular Dichroism and the Conformational Analysis of Biomolecules (Fasman, G. D., ed.), Plenum, New York, pp. 25–67.
Zemser, M., Friedman, M., Katzhendler, J., Greener, L. L., Minsky, A., and Gorinstein, S. (1994). J. Protein Chem. 13: 261–274.
Author information
Authors and Affiliations
Corresponding author
Additional information
Deceased December 8, 2001
Rights and permissions
About this article
Cite this article
McKenzie, H.A., Frier, R.D. Behavior of S1- and S2-Ovalbumin and S-Ovalbumin A1 in Urea Solution: Kinetics and Equilibria. J Protein Chem 22, 215–220 (2003). https://doi.org/10.1023/A:1025028705586
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1025028705586