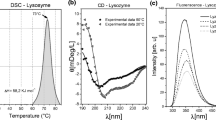
Abstract
A thermodynamic method is reported to monitor the conformational stability of lysozyme and ovalbumin in the presence of various cosolvents. Heats of dilution of the proteins in concentrated aqueous solutions of urea, ethanol or glucose have been determined at 298.15 K by flow microcalorimetry. The pairwise enthalpic interaction coefficients of the proteins in the different solvent media are derived: They allow to gain information about the influence of the cosolvents on the interactions between two interacting hydrated molecules of a protein, hence on its conformational stability. The two proteins behave very differently in the various cosolvents. In glucose, the coefficients for ovalbumin are positive up to 3 mol kg−1 of cosolvent and then negative, while those for lysozyme are negative up to 6 mol kg−1. In urea, coefficients for ovalbumin are positive, while negative for lysozyme up to 7 mol kg1. In ethanol, coefficients for ovalbumin are almost invariant, even at the highest concentrations of cosolvent, underlining that the hydration shell of the protein is such to maintain essentially unaltered the native conformation. For lysozyme, coefficients are negative and almost invariant up to 20 mol kg−1 ethanol: Then, a jump occurs toward much more large and negative values. The observed behaviors are rationalized also on the basis of the results previously obtained for small model molecules in concentrated solutions of urea, ethanol or glucose. The differences between the two proteins are explained in terms of the effects of the cosolvents on hydrophilic and hydrophobic interactions and account for the structural characteristics of each protein. In fact, notwithstanding both are globular proteins, they are differently packed and that could make them to react differently toward the action of a given cosolvent.



Similar content being viewed by others
References
Cinelli S, De Francesco A, Onori G, Paciaroni A. Thermal stability and internal dynamics of lysozyme as affected by hydration. Phys Chem Chem Phys. 2004;6:3591–5.
Roh JH, Curtis JE, Azzam S, Novikov VN, Peral I, Chowdhuri Z, Gregory RB, Sokolov AP. Influence of hydration on the dynamics of lysozyme. Biophys J. 2006;91:2573–88.
Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci USA. 2006;104:13997–4002.
England JL, Hilan G. Role of solvation effect in protein denaturation: from thermodynamics to single molecules and back. Annu Rev Phys Chem. 2011;62:257–77.
Lindgren M, Sparrman T, Westlund PO. A combined molecular dynamic simulation and urea 14N NMR relaxation study of the urea-lysozyme system. Spectrochim Acta Part A. 2010;75:953–9.
Panuszko A, Bruzdziak P, Zielkiewicz J, Wyrzykowski D, Stangret J. Effects of urea and trimethylamine-N-oxide on the properties of water and the secondary structure of hen egg white lysozyme. J Phys Chem. 2009;113:14797–809.
Lehmann MS, Mason SA, McIntyre GJ. Study of ethanol–lysozyme interactions using neutron diffraction. Biochemistry. 1985;24:5862–9.
Kaushik JK, Bhat R. Thermal stability of proteins in aqueous polyol solutions: role of the surface tension of water in the stabilizing effect of polyols. J Phys Chem B. 1998;102:7058–66.
Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979;18:5191–6.
Uedaira H, Uedaira H. The effect of sugars on the thermal denaturation of lysozyme. Bull Chem Soc Jpn. 1980;53:2451–5.
Fujita Y, Noda Y. Differential scanning calorimetric studies on the thermal denaturation of ribonuclease A in aqueous 2-methyl-2,4-pentanediol. Bull Chem Soc Jpn. 1984;57:1891–6.
Fujita Y, Noda Y. The effect of ethylene glycol on the thermal denaturation of ribonuclease A and chymotrypsinogen A as measured by differential scanning calorimetry. Bull Chem Soc Jpn. 1984;57:2177–83.
Karuppiah N, Sharma A. Cyclodextrins as protein folding aids. Biochem Biophys Res Commun. 1995;211:60–6.
Sharma L, Sharma A. Influence of cyclodextrin ring substituents on folding-related aggregation of bovine carbonic anhydrase. Eur J Biochem. 2001;268:2456–63.
Castronuovo G, Niccoli M. A calorimetric study of the interactions in the aqueous solutions of lysozyme in the presence of denaturing cosolvents. Thermochim Acta. 2012;543:254–9.
Franks F. Protein stability: the value of ‘old literature’. Biophys Chem. 2002;96:117–27.
Banipal TS, Singh G. Thermodynamic study of solvation of some amino acids, diglycine and lysozyme in aqueous and mixed aqueous solutions. Thermochim Acta. 2004;412:63–83.
Bolen DW, Rose GD. Structure and energetic of the hydrogen-bonded backbone in protein folding. Annu Rev Biochem. 2008;77:339–62.
Castronuovo G, d’Isanto G, Elia V, Velleca F. Role of cosolvent in hydrophobic interactions. Calorimetris studies of alkanols in concentrated aqueous solutions of urea at 298 K. J Chem Soc Faraday Trans. 1996;92:3087–91.
Castronuovo G, Elia V, Moniello V, Velleca F, Perez-Casas S. Effect of a cosolvent on the hydrophobic interactions. A calorimetric study of alkane-m, n-diols in concentrated aqueous solutions of ethanol. Phys Chem Chem Phys. 1999;1:1887–92.
Castronuovo G, Elia V, Niccoli M, Velleca F. Calorimetric studies of hydrophobic interactions of alkanols in concentrated aqueous solutions of glucose. Implications for the mechanism of protein stabilization by sugars. Thermochim Acta. 2002;389:1–9.
Castronuovo G, Elia V, Postiglione C, Velleca F. Interactions of aminoacids in concentrated aqueous solutions of urea or ethanol. Implications for the mechanism of protein denaturation. Thermochim Acta. 1999;339:11–9.
Mc Millan WG Jr, Mayer JE. The statistical thermodynamics of multicomponent systems. J Chem Phys. 1945;13:276–305.
Kozak JJ, Knight WS, Kauzmann W. Solute–solute interactions in aqueous solutions. J Chem Phys. 1968;48:675–90.
Friedman HL, Krishnan CV. Studies of hydrophobic bonding in aqueous alcohols. Enthalpy measurements and model calculations. J Solut Chem. 1973;2:119–40.
Franks F, Pedley MD, Reid DS. Solute interactions in dilute aqueous solutions. Part 1. Microcalorimetric study of the hydrophobic interaction. J Chem Soc Faraday Trans I. 1976;72:359–67.
Franks F, Pedley MD. Solute interactions in dilute aqueous solutions. Part 5. Microcalorimetric study of polyols and their mixtures with alkanols. J Chem Soc Faraday Trans I. 1983;79:2249–60.
Shimizu S. Estimation of excess solvation numbers of water and cosolvents from preferential interaction and volumetric experiments. J Chem Phys. 2004;120:4989–90.
Shimizu S, Matubayasi N. Preferential hydration of proteins: a Kirkwood–Buff approach. Chem Phys Lett. 2006;420:518–22.
Timasheff SN. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc Natl Acad Sci USA. 2002;99:9721–6.
Chipman DM, Sharon N. Mechanism of lysozyme action. Science. 1969;165:454–65.
Timasheff SN, Xie G. Preferential interaction of urea with lysozyme and their linkage to protein denaturation. Biophys Chem. 2003;105:421–48.
Andini S, Castronuovo G, Elia V, Pignone A, Velleca F. Chiral recognition in aqueous solutions: on the role of urea in hydrophobic interactions of unsubstituted α-amino acids. J Solut Chem. 1996;25:837–48.
Castronuovo G, Elia V, Velleca F. Hydrophilic interactions determine cooperativity of hydrophobic interactions and molecular recognition in aqueous solutions of non electrolytes. The preferential configuration model. Curr Top Solut Chem. 1997;2:125–42.
Korolev VP, Antonova OA, Smirnova NL. Thermal properties and interpartical interactions of l-proline, glycine, and l-alanine in aqueous urea solutions at 288–318 K. J Therm Anal Calorim. 2012;108:1–7.
Okamoto B, Wood RH, Thompson PT. Freezing points of aqueous alcohols. Free energy of interaction of the CHOH, CH2, CONH and C=C functional groups in dilute aqueous solutions. J Chem Soc Faraday Trans I. 1978;74:1990–2007.
Auton M, Holthauzen LM, Bolen DW. Anatomy of energetic changes accompanying urea-induced protein denaturation. Proc Natl Acad Sci USA. 2007;103:15317–22.
McKenzie HA, Frier RD. The behavior of R-ovalbumin and its individual components A1, A2, and A3 in urea solution: kinetics and equilibria. J Protein Chem. 2003;22:207–14.
Kamiyama T, Liu HL, Kimura T. Preferential solvation of lysozyme by dimethylsulfoxide in binary solutions of water and dimethylsulfoxide. J Therm Anal Calorim. 2009;95:353–9.
Ortore MG, Mariani P, Carsughi F, Cinelli S, Onori G, Teixeira J, Spinozzi F. Preferential solvation of lysozyme in water/ethanol mixtures. J Chem Phys. 2011;135:245103–12.
Sirotkin VA, Khadiullina AV. A study of the hydration of ribonuclease A using isothermal calorimetry. Effect of the protein hydrophobicity and polarity. J Therm Anal Calorim. 2014;118:951–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niccoli, M., Castronuovo, G. The conformational stability of ovalbumin and lysozyme in the aqueous solutions of various cosolvents. J Therm Anal Calorim 123, 2149–2156 (2016). https://doi.org/10.1007/s10973-015-4921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4921-5