Abstract
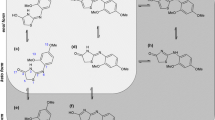
Some diastereomerically pure 4,6-bis-(phenoxymethyl)-l,2,5-trithiepanes were synthesized and unambiguously assigned. Their conformational properties and dynamic behavior were investigated by various NMR spectroscopic methods and quantum-chemical calculations at the HF/6-31G* level. The ground states of these compounds proved to be twist-chairs. A ring interconversion can occur in the meso-isomers as well as in the (±)-isomers. This interconversion can be described as a simultaneous inversion of the disulfide bridge. In the case of the meso-isomers, both ground states are mirror images of each other and the transition state is a highly symmetrical chair. The barrier heights of interconversion were determined to be in the range of 50 kJ/mol by variable-temperature NMR measurements. The ground states as well as the transition state of the (±)-isomers were found to be nonsymmetrical. However, those dynamic processes that are fast with respect to the NMR time scale lead to averaged NMR spectra at room temperature. A further dynamic process found through the quantum-chemical calculations is a “flapping” of the meth-ylene groups of the rings. The energy barrier of this flapping was calculated to be very small (< 20 kJ/mol) and could not be observed by low-temperature NMR measurements.
Similar content being viewed by others
REFERENCES
Heinicke, J. Dissertation, Fakultät für Chemie und Mineralogie der Universität Leipzig, 1994. (b) Heinicke, J.; Ristau, T.; Jelonek, S.; Mühlstädt, M. J. Prakt. Chem. 1996, 338, 327.
Houk, J.; Whitesides, G. M. J. Am. Chem. Soc. 1987, 109, 6825.
Field, L.; Foster, C. H. J. Org. Chem. 1970, 35, 479.
Goodrow, M. H.; Musker, W. K. Synthesis 1981, 457.
Musorin, G. K.; Amosova, S. W. Zh. Obshch. Khim. 1987, 10, 2253.
Chernyshev, E. A.; Kuz'min, O. V.; Lebedev, A. V.; Gusev, A. I.; Kirillova, N. I.; Nametkin, N. S.; Tyurin, V. D.; Krapivin, A. M.; Kubasova, N. A. J. Organomet. Chem. 1983, 252, 133.
Setzer, W. N.; Glass, R. S. In Conformational Analysis of Medium-Sized Heterocycles, Glass, R. S., ed.; VCH Verlagsgesellschaft mbH: Weinheim, 1988, pp. 151ff.
Oki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry; VCH: Deerfield Beach, 1985, pp. 307ff.
Li, F.; Cui, W.; Allinger, N. L. J. Comput. Chem. 1994, 15, 769.
Menard, D.; St-Jacques, M. Can. J. Chem. 1986, 64, 2142.
Chenard, B. L.; Dixon, D. A.; Harlow, R. L.; Rose, D. C.; Fukunaga, T. J. Org. Chem. 1987, 52, 2411.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Gill, P. M. W.; Johnson, B. G.; Robb, M. A.; Cheeseman, J. R.; Keith, T.; Petersson, G. A.; Montgomery, J. A.; Raghavachari, K.; Al-Laham, M. A.; Zakrzewski, V. G.; Ortiz, J. V.; Foresman, J. B.; Cioslowski, J.; Stefanow, B. B.; Nanayakkara, A.; Challacombe, M.; Peng, C. Y.; Ayala, P. Y.; Chen, W.; Wong, M. W.; Andres, J. L.; Replogle, E. S.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Binkley, J. S.; Defrees, D. J.; Baker, J.; Stewart, J. J. P.; Head-Gordon, M.; Gonzales, C.; Pople, J. A. Gaussian 94, Revision C.1; Gaussian Inc.: Pittsburgh, Pennsylvania, 1995.
Peng, C.; Schlegel, H. B. Isr. J. Chem. 1993, 33, 449.
Peng, C.; Ayala, P. Y.; Schlegel, H. B.; Frisch, M. J. J. Comp. Chem. 1996, 17, 49.
Backer, J.; Hehre, W. J. J. Comp. Chem. 1991, 12, 606.
Bearpark, M. J.; Robb, M. A.; Schlegel, H. B. Chem. Phys. Lett. 1994, 223, 269.
Sheldrick, G. M.; SHELXS-86: Program for Crystal Structure Solution, Universität Göttingen, 1986.
Sheldrick, G. M.; SHELXL-93: Program for Crystal Structure Determination, Universität Göttingen, 1993.
Laatikainen, R.; Niemitz, M.; Weber, U.; Sundelin, J.; Hassinen, T.; Vepsäläinen, J. J. Magn. Reson. A 1996, 120, 1.
Günther, H. NMR-Spektroskopie, 3rd ed.; Georg Thieme Verlag: Stuttgart, 1992.
Entrena, A.; Campos, J.; Gomez, J. A.; Gallo, M. A.; Espinosa, A. J. Org. Chem. 1997, 62, 337.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heydenreich, M., Koch, A., Ristau, T. et al. Conformational Analysis of Sulfur-Containing Heterocycles, Part I. Synthesis and Structural Determination of Diastereomerically Pure 4,6-Bis-(phenoxymethyl)-1,2,5-trithiepanes. Structural Chemistry 9, 139–148 (1998). https://doi.org/10.1023/A:1022416021618
Issue Date:
DOI: https://doi.org/10.1023/A:1022416021618