Abstract
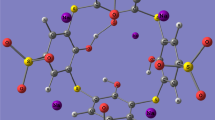
Para-tert-butylthiacalix[4]arenimines with mono- and distally substituted functional groups have been examined in terms of their structure and spectra. The structure and H-bonds of these compounds can be studied by comparing their vibrational and NMR spectra. The spectra of several conformations of the molecules TCA1-4 were calculated. For the molecules TCA3 and TCA4, the most stable conformation is a distorted cone (DC2) with the same imine or phthalimide group orientation, consequently. The least stable conformation is pinched cone (PC). The conformations of molecules TCA2, TCA3, and TCA4 are DC1 and DC2, respectively. H-bonds in the molecules TCA1-4 alter their supramolecular properties. Ionization energy and dipole moment decrease as monosubstituted thiacalixarene (TCA1) transforms into disubstituted TCA2. In this instance, there is an increase in softness, electrophilicity, chemical potential, and electron affinity. An increase in the number of methylene groups in disubstituted thiacalixarenes from TCA4 to TCA2 is accompanied by an increase in ionization energy, electron affinity, and electrophilicity.







Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
Code availability
NA.
References
Gutsche CD (2008) Calixarenes: an introduction, 2nd edn. The Royal Society of Chemistry, Cambridge, UK
Vicens J, Harrowfield J, Baklouti L (eds) (2007) Calixarenes in the nanoworld. Springer, Dordrecht
Neri P, Sessler J, Wang M-X (eds) (2016) Calixarenes and beyond. Springer International Publishing, Switzerland
Ovsyannikov AS, Solovieva SE, Antipin IS, Ferlay S (2017) Coordination polymers based on calixarene derivatives: structure and properties. Coord Chem Rev 352:151–186. https://doi.org/10.1016/j.ccr.2017.09.004
Solovieva SE, Burilov VA, Antipin IS (2017) Thiacalix[4]arene’s lower rim derivatives: synthesis and supramolecular properties. Macroheterocycles 10:134–146. https://doi.org/10.6060/mhc170512a
Asfari Z, Bohmer V, Harrowfield J, Vincens J (2001) Calixarenes. Kluwer, Netherlands
Perret F, Coleman AW (2011) Biochemistry of anionic calix[n]arenes. Chem Commun 47:7303–7319. https://doi.org/10.1039/C1CC11541C
Perret F, Lazar AN, Coleman AW (2006) Biochemistry of the para-sulfonato-calix[n]arenes. Chem Commun 42:2425–2438. https://doi.org/10.1039/B600720C
Ludwig R, Calixarenes for biochemical recognition and separation, (2005) Microchim. Acta 152:1–19. https://doi.org/10.1007/s00604-005-0422-8
Paclet MN, Rousseau CF, Yannick C, Morel F, Coleman AW (2006) An absence of non-specific immune response towards para-sulphonato-calixarenes. J Inclusion Phenom Macrocyclic Chem 55:353–357. https://doi.org/10.1007/s10847-006-9107-0
Coleman AW, Jebors S, Cecillon S, Perret P, Garrin D, Marti-Battle D, Moulin M (2008) Toxicity and biodistribution of para-sulfonatocaix[4]arene in mice, New. J Chem 32:780–782. https://doi.org/10.1039/B718962A
Mokhtari B, Pourabdollah K, Dallali N (2011) A review of calixarene applications in nuclear industries. J Radioanal Nucl Chem 287:921–934. https://doi.org/10.1007/s10967-010-0881-1
Reinhoudt DN (1996) Transduction of molecular recognition into electronic signals. Recl Trav Chim Pays-Bas 115:109–118. https://doi.org/10.1002/recl.19961150202
Kenis PJA, Noordman OFJ, Houubrechts S, Engbersen GFJ, Persoons A, van Hulst NF, Reinhoudt DN (1998) Second-order nonlinear optical properties of the four tetranitrotetrapro-poxycalix[4]arene conformers. J Am Chem Soc 120:7875–7883. https://doi.org/10.1021/ja980791t
Swager TM, Xu B (1994) Liquid crystalline calixarenes. J Incl Phenom 19:389–398. https://doi.org/10.1007/BF00708995
Shinkai S (1991) Functionalized calixarenes: new applications as catalysts, ligands, and host molecules. Top Inclusion Sci 3:173–198. https://doi.org/10.1007/978-94-009-2013-2-7
Zabala-Lekuona A, Manuel Seco J, Colacio E (2021) Single-molecule magnets: from Mn12-ac to dysprosium metallocenes, a travel in time. Coord Chem Rev 441:213984. https://doi.org/10.1016/j.ccr.2021.213984
Aldoshin S, Antipin I, Ovcharenko V, Solov’eva S, Bogomyakov A, Korchagin A, Shilov G, Yur’eva EB, Mushenok FV, Bozhenko K, Utenyshev A (2013) Synthesis, structure, and properties of a new representative of the family of calix[4]arene-containing (MnII2MnIII2)-clusters. Russ Chem Bull 62:536–542. https://doi.org/10.1007/s11172-013-0074-5
Kumar R, Lee YO, Bhalla V, Kumar M, Kim JS (2014) Recent developments of thiacalixarene molecular motifs. Chem Soc Rev 43:4824–4870. https://doi.org/10.1039/c4cs00068d
Mohammed-Ziegler I, Billes F (2007) Optical spectroscopy and theoretical studies in calixarene chemistry. J Incl Phenom 58:19–42. https://doi.org/10.1007/s10847-006-9132-z
Furer VL, Borisoglebskaya EI, Zverev VV, Kovalenko VI (2006) DFT and IR spectroscopic analysis of p-tert-butylcalix[4]arene. Spectrochim Acta 63:207–212. https://doi.org/10.1016/j.saa.2005.05.006
Kovalenko VI, Chernova AV, Borisoglebskaya EI, Katsyuba SA, Zverev VV, Shagidullin RR, Antipin IS, Solovieva SE, Stoikov II, Konovalov AI (2002) Cooperative intramolecular hydrogen bond and conformations of thiacalix[4]arene molecules. Russ Chem Bull 51:825–827. https://doi.org/10.1023/A:1016084817527
Furer VL, Potapova LI, Vatsouro IM, Kovalev VV, Shokova EA, Kovalenko VI (2019) Study of conformation and hydrogen bods in the p-1-adamantylcalix[8]arene by IR spectroscopy and DFT. J Incl Phenom 95:63–71. https://doi.org/10.1007/s10847-019-00916-8
Furer VL, Vandyukov AE, Popova EV, Solovieva SE, Antipin IS, Kovalenko VI (2020) Vibrational spectra study of p-sulfonatocalix[4]arene containing azobenzene groups, J. Mol. Struct. 1200:№ 127058. https://doi.org/10.1016/j.molstruc.2019.127058
Furer VL, Vandyukov AE, Kleshnina SR, Solovieva SE, Antipin IS, Kovalenko VI (2020) FT-IR and FT-Raman study of p-sulfonatocalix[8]arene, J. Mol. Struct. 1203: № 127474. https://doi.org/10.1016/j.molstruc.2019.127474
Solovieva SE, Popova EV, Omran AO, Gubaidullin AT, Kharlamov SV, Latypov ShK, Antipin IS, Konovalov AI (2011) Unusual functionalization of lower rim of thiacalix[4]arene: competition of alkylation and transalkylation. Russ Chem Bull Int Ed 60:486–498. https://doi.org/10.1007/s11172-011-0076-0
Latypov ShK, Kharlamov SV, Muravev AA, Balandina AA, Solovieva SE, Antipin IS, Konovalov AI (2013) Conformational diversity and dynamics of distally disubstituted calix and thiacalix[4]arenes in solution. J Phys Org Chem 26:407–414. https://doi.org/10.1002/poc.3101
Iki N, Kabuto C, Fukushima T, Kumagai H, Takeya H, Miyanari S, Miyashi T, Miyano S (2000) Synthesis of p-tert-butylthiacalix[4]arene and its inclusion property. Tetrahedron 56:1437–1443. https://doi.org/10.1016/S0040-4039(99)01503-8
Bhalla V, Kumar M, Hattori T, Miyano S (2004) Stereoselective synthesis of all stereoisomers of vicinal and distal bis (O-2-aminoethyl)-p-tert-butylthiacalix [4] arene. Tetrahedron 60(28):5881–5887. https://doi.org/10.1016/j.tet.2004.05.035
Stoikov I, Galukhin A, Zaikov E, Antipin S (2009) Synthesis and complexation properties of 1,3-alternate stereoisomers of p-tert-butylthiacalix[4]arenes tetrasubstituted at the lower rim by the phthalimide group. Mendeleev Commun 19(4):193–195. https://doi.org/10.1016/j.mencom.2009.07.006
Liang LI, Wei-wei GU, Chao-guo YAN (2010) Syntheses, crystal structures and complexing properties of 1,3-distal calix[4]arene Schiff bases. Chem Res Chinese Universities 26:38–45
Laikov DN, Ustynyuk YA (2005) PRIPODA-04: a quantum-chemical program suite. New possibilities in the study of molecular systems with the application parallel computing. Russ Chem Bull Int Ed 54:821–826. https://doi.org/10.1007/s11172-005-0329-x
Jamroz MH (2013) Vibrational energy distribution analysis (VEDA): scopes and limitations. Spectrochim Acta 114:220–230. https://doi.org/10.1016/j.saa.2013.05.096
Wolinsky K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–82260. https://doi.org/10.1021/ja00179a005
Ugozzoli F, Andretti GD (1992) Symbolic representation of the molecular conformation of calixarenes. J Incl Phenom 13:337–348. https://doi.org/10.1007/BF01133233
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Comput Mol Sci 2:1–42. https://doi.org/10.1002/wcms.51
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Acknowledgements
The authors are grateful to the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for technical assistance in research.
Funding
This work was supported by the Russian Science Foundation (Project N 22–73-10139).
Author information
Authors and Affiliations
Contributions
V.F.: conceptualization, methodology, software, writing–original draft preparation, and editing. A.V.: investigation of IR and Raman spectra. A.O., A.A., and I.S.: synthesis of calixarenes. S.S.: conceptualization, methodology, reviewing, and editing. I.A.: conceptualization, methodology, reviewing, and editing.
Corresponding author
Ethics declarations
Ethics approval
The authors agree with ethical standards.
Consent to participate
The authors agree to participate in the article.
Consent for publication
The authors agree to participate in the publication of the article.
Competing interests
The authors declare no competing interests.
Additional information
This work was not published previously, and is not submitted to more than one journal. No data have been fabricated or manipulated.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furer, V.L., Vandyukov, A.E., Ovsyannikov, A.S. et al. Study of the structure of 1,3-disubstituted thiacalix[4]arenes with phthalimide and imine groups using vibrational and NMR spectroscopy. Struct Chem (2024). https://doi.org/10.1007/s11224-024-02298-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-024-02298-1