Abstract
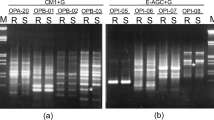
Meloidogynejavanica is the most widely spread nematode pest on soybean in SouthAfrica. Only a few registered commercial South African cultivars are poor hostsof this nematode species and there is an urgent need for an efficient breedingprogramme for resistant cultivars of all maturity groups. However, breeding ishampered by laborious screening procedures for selection of poor host cultivarsand/or lines. The objective of this study was to develop an economically viablemolecular marker system for application in selection procedures. BothRestriction Fragment Length Polymorphism (RFLP) and Amplified Fragment LengthPolymorphism (AFLP) screening techniques identified markers linked togall-indexvariation in a segregating population of 60 F2 progeny from a crossbetween a resistant cultivar (Gazelle) and a highly susceptible variety(Prima).A codominant RFLP marker( B212) was linked significantly to M.javanica resistance and explained 62% of the variation ingall-index.Seven AFLP markers were linked significantly to the resistance trait, of whichfour were linked in repulsion phase and three in coupling phase. All seven AFLPmarkers mapped to LG-F (Linkage Group F) on the public soybean molecular map.The major quantitative trait locus (QTL) for resistance mapped between markersE-ACC/M-CTC2(SOJA6) (linked in coupling phase), B212 and E-AAC/M-CAT1(SOJA7)(linked in repulsion phase). These two AFLP markers bracketing the majorresistance QTL were successfully converted to SCARs (Sequence CharacterizedAmplified Regions). Marker E-ACC/M-CTC2 was converted to a codominant SCARmarker SOJA6, which accounted for 41% of variation in gall-index in the mappingpopulation. Marker E-AAC/M-CAT1 was converted to a dominant SCAR marker (SOJA7)and explained 42% of gall-index variation in the mapping population. These twomarkers mapped approximately 3.8 cM and 2.4 cMrespectively from the resistance QTL. This study represents the first report ofthe development of PCR-based sequence specific markers linked to M.javanica resistance in soybean.
Similar content being viewed by others
References
Bai D., Reeleder R. and Brandle J.E. 1995. Identification of two RAPD markers tightly linked with the Nicotiana debneyi gene for resistance to black root rot of tobacco. Theor. and Appl. Genet. 91: 1184–1189.
Cho Y.G., Blair W.M., Panaud O. and McCouch S.R. 1996. Cloning and mapping of variety-specific rice genomic DNA sequences: amplified fragment length polymorphisms (AFLP) from silver-stained polyacrylamide gels. Genome. 39: 373–378.
Dellaporta S.L., Wood J. and Hicks J.B. 1983. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1: 19–20.
Fourie H., McDonald A.H. and Loots G.C. 1999. Host suitability of South African commercial soybean cultivars to two root-knot nematode species and races. African Plant Protection 2: 119–124.
Giovannoni J.J., Wing R.A., Ganal M.W. and Tanksley S.D. 1991. Isolation of molecular markers from specific chromosomal intervals using DNA pools from existing mapping populations. Nucl. Acids Res. 19: 6553–6558.
Haley S.D., Afanador L. and Kelly J.D. 1994. Selection for monogenic pest resistance with coupling-and repulsion-phase RAPD markers. Crop Sci. 34: 1061–1066.
Hussey R.S. and Boerma H.R. 1981. A greenhouse screening procedure for root-knot nematode resistance in soybeans. Crop Sci. 21: 794–796.
Johnson E., Miklas P.N., Stavely J.R. and Martinez-Cruzado J.C. 1995. Coupling-and repulsion-phase RAPDs for marker-assisted selection of PI 181996 rust resistance in common bean. Theor. and Appl. Gen. 90: 659–664.
Kleynhans K.P.N. 1991. The Root-knot Nematodes of South Africa, Technical communication–Department of Agricultural Development, no. 231.
Lincoln S., Daly M. and Lander E. 1992. Mapping genes controlling quantitative traits with MAPMAKER/QTL 1.1. 2nd edn, Whitehead Institute Technical Report.
Luzzi B.M., Boerma H.R. and Hussey R.S. 1987. Resistance to three species of root-knot nematode in soybean. Crop Sci. 27: 258–262.
Luzzi B.M., Tamulonis J.P., Hussey R.S. and Boerma H.R. 1995. Inheritance of resistance to the Javanese root-knot nematode in soybean. Crop Sci. 35: 1372–1375.
Meksem K., Leister D., Peleman J., Zabeau M., Salamini F. and Gebhardt C. 1995. A high-resolution map of the vicinity of the R1 locus on chromosome V of potato based on RFLP and AFLP Markers. Mol. Gen. Genet. 249: 74–81.
Michelmore R.W., Paran I. and Kesseli R.V. 1991. Identification of markers linked to disease resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions using segregating populations. Proc. Natl. Acad. of Sci. USA 88: 9828–9832.
Miklas P.N., Johnson E., Stone V., Beaver J.S., Montoya C. and Zapata M. 1996. Selective mapping of QTL conditioning disease resistance in common bean. Crop Sci. 36: 1344–1351.
Qu L.J., Foote T.N., Roberts M.A., Aragon-Alcaide L., Snape J.W. and Moore G. 1998. A simple PCR-based method for scoring the ph1b deletion in wheat. Theor. and Appl. Genet. 96: 371–375.
Riekert H.F. 1995. An adapted method for extraction of root-knot nematode eggs from maize root samples. African Plant Protection 1: 41–43.
Shan X., Blake T.K. and Talbert L.E. 1999. Conversion of AFLP markers to sequence-specific PCR markers in barley and wheat. Theor. and Appl. Genet. 98: 1072–1078.
Southern E.M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. of Mol. Biol. 98: 503–517.
Tamulonis J.P., Luzzi B.M., Hussey R.S., Parrott W.A. and Boerma H.R. 1997. DNAmarkers associated with resistance to Javanese root-knot nematode in soybean. Crop Sci. 37: 783–788.
Zabeau M. and Vos P. 1993. Selective restriction fragment ampli-fication: a general method for DNA fingerprinting., European Patent Application 92402629.7 (Publ. No. 0 534 858 A1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mienie, C., Fourie, H., Smit, M. et al. Identification of AFLP markers in soybean linked to resistance to Meloidogyne javanica and conversion to Sequence Characterized Amplified Regions (SCARs). Plant Growth Regulation 37, 157–166 (2002). https://doi.org/10.1023/A:1020585023976
Issue Date:
DOI: https://doi.org/10.1023/A:1020585023976