Abstract
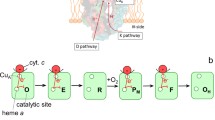
Novel experimental evidence is presented further supporting the hypothesis that, starting with resting oxidized cytochrome c oxidase, the internal electron transfer to the oxygen binding site is kinetically controlled. The reduction of the enzyme was followed spectroscopically and in the presence of NO or CO, used as trapping ligands for reduced cytochrome a3; ruthenium hexamine was used as a spectroscopically silent electron donor. Consistent with the high combination rate constant for reduced cytochrome a3, NO proved to be a very efficient trapping ligand, while CO did not. The results are discussed in view of two alternative (thermodynamic and kinetic) hypotheses of control of electron transfer to the binuclear (cyt.a3-CuB) center. Fulfilling the prediction of the kinetic control hypothesis: i) the reduction of cytochrome a3 and ligation are synchronous and proceed at the intrinsic rate of cytochrome a3 reduction, ii) the measured rate of formation of the nitrosyl derivative is independent of the concentration of both the reductant and NO.
Similar content being viewed by others
REFERENCES
Gibson, Q., Greenwwod, C., Wharton, D. C., and Palmer G. (1965). “The reactions of cytochrome oxidase with cytochrome c,” J. Biol. Chem. 240, 888-894.
Sarti, P., Antonini, G., Malatesta, F., Vallone, B., and Brunori, M. (1988). ‘Is the internal electron transfer the rate-limiting step in the catalytic cycle of cytochrome c oxidase?”, Ann. N.Y Acad. Sci. 550, 161-166.
Malatesta, F., Sarti, P., Antonini, G., Vallone, B., and Brunori, M. (1990). “Electron transfer to the binuclear center in cytochrome oxidase: catalytic significance and evidence for an additional intermediate,” Proc. Natl. Acad. Sci USA 87, 7410-7413.
Han, S., Ching, Y., and Rousseau, D. (1990). “Primary intermediate in the reaction of oxygen with fully reduced cytochrome c oxidase,” Proc. Natl. Acad. Sci USA 87, 2491-2495.
Oliveberg, M., and Malmström, Bo, G. (1991). “Internal electron transfer in cytochrome c oxidase: evidence for a rapid equilibrium between cytochrome a and the bimetallic site,” Biochemistry 30, 7053-7057.
Verkhosvky, M. I., Morgan, J. E., and Wikström, M. (1994). “Oxygen binding and activation: early steps in the reaction of oxygen with cytochrome c oxidase,” Biochemistry 33, 3079-3086.
Woodruff, W. H. (1993). “Coordination dynamics of hemecopper oxidases. The ligand shuttle and the control and coupling of electron transfer and proton translocation,” J. Bioenerg. Biomembr., 25, 177-188.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomikazi, T., Yamaguchi, H., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1995). “Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 Å,” Science 269, 1069-1074.
Verkhovsky, M. I., Morgan, J. E., and Wikström, M. (1995). “Control of electron delivery to the oxygen reduction site of cytochrome c oxidase: a role for protons,” Biochemistry 34, 7483-7491.
Palmer, G., Baker, G. M., and Noguchi, M. (1988). “The rapid and slow forms of cytochrome oxidase,” Chem. Scr. 28A, 41-46.
Moody, A. J. (1996). “As prepared' forms of fully oxidised haem/Cu terminal oxidases. Review,” Biochim. Biophys. Acta 1276, 6-20.
Soulimane, T., and Buse, G. (1995). “Integral cytochrome c oxidase: preparation and progress towards a three-dimensional crystallization,” Eur. J. Biochem. 227, 588-595.
Blackmore, R. S., Greenwood, C., and Gibson, Q. H. (1991). “Studies of the primary oxygen intermediate in the reaction of fully reduced cytchrome oxdase,” J. Biol. Chem. 266, 19245-19249.
Giuffrè, A., Sarti, P., D'Itri, E., Buse, G., Soulimane, T., and Brunori, M. (1996). “On the mechanism of inhibition of cytochrome c oxidase by nitric oxide,” J. Biol. Chem. 271, 33404-33408.
Gibson Q. H., and Greenwood, C. (1963). “Reactions of cytochrome oxidase with oxygen and carbon monoxide,” Biochem. J. 86, 541-555.
Brunori, M., Giuffré, A., D'Itri, E., and Sarti, P. (1997). “Internal electron transfer in Cu-heme oxidases: thermodynamic or kinetic control?,” J. Biol. Chem. 272, 19870-19874.
Blair, D. F., Ellis, W. R., Wang, H., Gray, H. B., and Chan, S. I. (1986). “Spectroelectrochemical study of cytochrome c oxidase: pH and temperature dependences of the cytochrome potentials,” J. Biol. Chem. 261, 11524-11537.
Hallen, S., Brzezinski, P., and Malmsrom B. G. (1994). “Internal electron transfer in cytochrome c oxidase is coupled to the protonation of a group close to the bimetallic site,” Biochemistry 33, 1467-1472
Brunori, M., Antonini, G., Giuffrè, A., Malatesta, F., Nicoletti, F., Sarti, P., and Wilson, M. T. (1994). “Electron transfer and ligand binding in terminal oxidases: impact of recent structural information,” FEBS Lett. 350, 164-168.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brunori, M., Giuffré, A., Malatesta, F. et al. Investigating the Mechanism of Electron Transfer to the Binuclear Center in Cu-Heme Oxidases. J Bioenerg Biomembr 30, 41–45 (1998). https://doi.org/10.1023/A:1020503410377
Issue Date:
DOI: https://doi.org/10.1023/A:1020503410377