Abstract
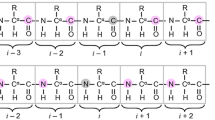
A triple-resonance pulse scheme is described which records15N, NH correlations of residues that immediately follow amethyl-containing amino acid. The experiment makes use of a15N, 13C and fractionally deuterated proteinsample and selects for CH2D methyl types. The experiment isthus useful in the early stages of the sequential assignment process as wellas for the confirmation of backbone 15N, NH chemical shiftassignments at later stages of data analysis. A simple modification of thesequence also allows the measurement of methyl side-chain dynamics. This isparticularly useful for studying side-chain dynamic properties in partiallyunfolded and unfolded proteins where the resolution of aliphatic carbon andproton chemical shifts is limited compared to that of amide nitrogens.
Similar content being viewed by others
References
Boyd, J. and Scoffe, N. (1989) J. Magn. Reson., 85, 406–413.
Dötsch, V., Matsuo, H. and Wagner, G. (1996a) J. Magn. Reson., B112, 95–100.
Dötsch, V., Oswald, R.E. and Wagner, G. (1996b) J. Magn. Reson., B110, 107–111.
Dötsch, V., Oswald, R.E. and Wagner, G. (1996c) J. Magn. Reson., B110, 304–308.
Farmer, B.T. and Venters, R. (1995) J. Am. Chem. Soc., 117, 4187–4188.
Fu, R. and Bodenhausen, G. (1995) Chem. Phys. Lett., 245, 415–420.
Gardner, K.H., Konrat, R., Rosen, M.K. and Kay, L.E. (1996) J. Biomol. NMR, 8, 351–356.
Gardner, K.H. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 7599–7600.
Geen, H. and Freeman, R. (1991) J. Magn. Reson., 93, 93–141.
Gehring, K. and Guittet, E. (1995) J. Magn. Reson., B109, 206–208.
Grzesiek, S., Anglister, J. and Bax, A. (1993a) J. Magn. Reson., B101, 114–119.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 4369–4370.
Grzesiek, S. and Bax, A. (1993a) J. Biomol. NMR, 3, 185–204.
Grzesiek, S. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 12593–12594.
Johnson, P.E., Joshi, M.D., Tomme, P., Kilburn, D.G. and McIntosh, L.P. (1996a) Biochemistry, 35, 14381–14394.
Johnson, P.E., Tomme, P., Joshi, M.D. and McIntosh, L.P. (1996b) Biochemistry, 35, 13895–13906.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Kay, L.E. (1993) J. Am. Chem. Soc., 115, 2055–2056.
Kay, L.E., Xu, G.Y. and Yamazaki, T. (1994) J. Magn. Reson., A109, 129–133.
Kay, L.E. and Gardner, K.H. (1997) Curr. Opin. Struct. Biol., in press.
Kupce, E. and Freeman, R. (1995) J. Magn. Reson., A115, 273–276.
Logan, T.M., Olejniczak, E.T., Xu, R. and Fesik, S.W. (1992) FEBS Lett., 314, 413–418.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
McCoy, M. and Mueller, L. (1992) J. Am. Chem. Soc., 114, 2108–2110.
McIntosh, L.P. and Dahlquist, F.W. (1990) Q. Rev. Biophys., 23, 1–38.
Metzler, W.J., Wittekind, M., Goldfarb, V., Mueller, L. and Farmer, B.T. (1996) J. Am. Chem. Soc., 118, 6800–6801.
Mohebbi, A. and Shaka, A.J. (1991) J. Chem. Phys., 178, 374–377.
Montelione, G.T., Lyons, B.A., Emerson, S.D. and Tashiro, M. (1992) J. Am. Chem. Soc., 114, 10974–10975.
Muhandiram, D.R., Yamazaki, T., Sykes, B.D. and Kay, L.E. (1995) J. Am. Chem. Soc., 117, 11536–11544.
Nietlispach, D., Clowes, R.T., Broadhurst, R.W., Ito, Y., Keeler, J., Kelly, M., Ashurst, J., Oschkinat, H., Domaille, P.J. and Laue, E.D. (1996) J. Am. Chem. Soc., 118, 407–415.
Olejniczak, E.T. and Fesik, S.W. (1994) J. Am. Chem. Soc., 116, 2215–2216.
Patt, S.L. (1992) J. Magn. Reson., 96, 94–102.
Rosen, M.K., Gardner, K.H., Willis, R.C., Parris, W.E., Pawson, T. and Kay, L.E. (1996) J. Mol. Biol., 263, 627–636.
Schleucher, J., Sattler, M. and Griesinger, C. (1993) Angew. Chem., Int. Ed. Engl., 32, 1489–1491.
Shaka, A.J., Keeler, T., Fenkiel, T. and Freeman, R. (1983) J. Magn. Reson., 52, 335–338.
Shortle, D. (1996) Curr. Opin. Struct. Biol., 6, 24–30.
Smith, B.O., Ito, Y., Raine, A., Teichmann, S., Ben-Tovim, L., Nietlispach, D., Broadhurst, R.W., Terada, T., Kelly, M., Oschkinat, K., Shibata, T., Yokoyama, S. and Laue, E.D. (1996) J. Biomol. NMR, {vn8}, 360–368.
Stonehouse, J., Shaw, G.L., Keeler, J. and Laue, E.D. (1994) J. Magn. Reson., B107, 178–184.
Venters, R.A., Farmer, B.T., Fierke, C.A. and Spicer, L.D. (1996) J. Mol. Biol., 264, 1101–1116.
Vuister, G.W. and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Yamazaki, T., Lee, W., Arrowsmith, C.H., Muhandiram, D.R. and Kay, L.E. (1994) J. Am. Chem. Soc., 116, 11655–11666.
Yang, D. and Kay, L.E. (1996) J. Magn. Reson., B110, 213–218.
Zhang, O., Forman-Kay, J.D., Shortle, D. and Kay, L.E. (1997a) J. Biomol. NMR, 9, 181–200.
Zhang, O., Kay, L.E., Shortle, D. and Forman-Kay, J.D. (1997b) J. Mol. Biol., in press.
Zhu, G. and Bax, A. (1990) J. Magn. Reson., 90, 405–410.
Zwahlen, C., Legault, P., Vincent, S.J.F., Greenblatt, J., Konrat, R. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 6711–6721.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muhandiram, D., Johnson, P.E., Yang, D. et al. Specific 15N, NH correlations for residues in15 N, 13C and fractionally deuterated proteins that immediately follow methyl-containing amino acids. J Biomol NMR 10, 283–288 (1997). https://doi.org/10.1023/A:1018301818803
Issue Date:
DOI: https://doi.org/10.1023/A:1018301818803