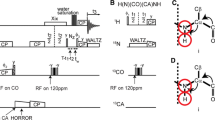
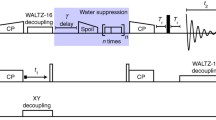
Abstract
Heteronucleus-detected dipolar based correlation spectroscopy is established for assignments of 1H, 13C, and 15N resonances and structural analysis in fully protonated proteins. We demonstrate that 13C detected 3D experiments are highly efficient and permit assignments of the majority of backbone resonances, as shown in an 89-residue dynein light chain 8, LC8 protein. With these experiments, we have resolved many ambiguities that were persistent in our previous studies using moderate MAS frequencies and lacking the 1H dimension. The availability of 1H isotropic chemical shifts measured with the heteronucleus-detected fast-MAS experiments presented here is essential for the accurate determination of the 1H CSA tensors, which provide very useful structural probe. Finally, our results indicate that 13C detection in fast-MAS HETCOR experiments may be advantageous compared with 1H detection as it yields datasets of significantly higher resolution in the 13C dimension than the 1H detected HETCOR versions.






Similar content being viewed by others
References
Agarwal V, Sardo M, Scholz I, Bockmann A, Ernst M, Meier BH (2013) PAIN with and without PAR: variants for third-spin assisted heteronuclear polarization transfer. J Biomol NMR 56(4):365–377
Akbey U, Lange S, Franks WT, Linser R, Rehbein K, Diehl A, van Rossum BJ, Reif B, Oschkinat H (2010) Optimum levels of exchangeable protons in perdeuterated proteins for proton detection in MAS solid-state NMR spectroscopy. J Biomol NMR 46(1):67–73
Asami S, Reif B (2013) Proton-detected solid-state NMR spectroscopy at aliphatic sites: application to crystalline systems. Acc Chem Res 46(9):2089–2097
Asami S, Rakwalska-Bange M, Carlomagno T, Reif B (2013) Protein-RNA Interfaces probed by 1H-detected MAS solid-state NMR spectroscopy. Angew Chem Int Ed 52(8):2345–2349
Barbar E (2008) Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry 47(2):503–508
Barbet-Massin E, Pell AJ, Knight MJ, Webber AL, Felli IC, Pierattelli R, Emsley L, Lesage A, Pintacuda G (2013) 13C-detected through-bond correlation experiments for protein resonance assignment by ultra-fast MAS solid-state NMR. ChemPhysChem 14(13):3131–3137
Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG (1995) Heteronuclear decoupling in rotating solids. J Chem Phys 103(16):6951–6958
Bermel W, Bertini I, Felli IC, Kummerle R, Pierattelli R (2003) 13C direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc 125(52):16423–16429
Bermel W, Bertini I, Felli IC, Kummerle R, Pierattelli R (2006a) Novel 13C direct detection experiments, including extension to the third dimension, to perform the complete assignment of proteins. J Magn Reson 178(1):56–64
Bermel W, Bertini I, Felli IC, Lee YM, Luchinat C, Pierattelli R (2006b) Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc 128(12):3918–3919
Bertini I, Luchinat C, Parigi G, Pierattelli R (2005) NMR spectroscopy of paramagnetic metalloproteins. ChemBioChem 6(9):1536–1549
Chevelkov V, Rehbein K, Diehl A, Reif B (2006) Ultrahigh resolution in proton solid-state NMR spectroscopy at high levels of deuteration. Angew Chem Int Ed 45(23):3878–3881
Clore GM, Gronenborn AM (1994) Multidimensional heteronuclear nuclear magnetic resonance of proteins. Methods Enzymol 239:349–363
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRpipe: a multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6(3):277–293
Ernst M, Samoson A, Meier BH (2001) Low-power decoupling in fast magic-angle spinning NMR. Chem Phys Lett 348(3–4):293–302
Hou GJ, Paramasivam S, Yan S, Polenova T, Vega AJ (2013) Multidimensional magic angle spinning NMR spectroscopy for site-resolved measurement of proton chemical shift anisotropy in biological solids. J Am Chem Soc 135(4):1358–1368
Hou GJ, Gupta R, Polenova T, Vega AJ (2014) A magic-angle-spinning NMR Spectroscopy method for the site-specific measurement of proton chemical-shift anisotropy in biological and organic solids. Isr J Chem 54(1–2):171–183
Lightcap CM, Sun S, Lear JD, Rodeck U, Polenova T, Williams JC (2008) Biochemical and structural characterization of the Pak1-LC8 interaction. J Biol Chem 283(40):27314–27324
Linser R, Dasari M, Hiller M, Higman V, Fink U, Lopez del Amo JM, Markovic S, Handel L, Kessler B, Schmieder P, Oesterhelt D, Oschkinat H, Reif B (2011) Proton-detected solid-state NMR spectroscopy of fibrillar and membrane proteins. Angew Chem Int Ed 50(19):4508–4512
Marchetti A, Jehle S, Felletti M, Knight MJ, Wang Y, Xu ZQ, Park AY, Otting G, Lesage A, Emsley L, Dixon NE, Pintacuda G (2012) Backbone assignment of fully protonated solid proteins by 1H detection and ultrafast magic-angle-spinning NMR spectroscopy. Angew Chem Int Ed 51(43):10756–10759
Marulanda D, Tasayco ML, McDermott A, Cataldi M, Arriaran V, Polenova T (2004) Magic angle spinning solid-state NMR spectroscopy for structural studies of protein interfaces. Resonance assignments of differentially enriched Escherichia coli thioredoxin reassembled by fragment complementation. J Am Chem Soc 126(50):16608–16620
Maudsley AA, Ernst RR (1977) Indirect detection of magnetic-resonance by heteronuclear 2-dimensional spectroscopy. Chem Phys Lett 50(3):368–372
McDermott A (2009) Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys 38:385–403
Reif B, Jaroniec CP, Rienstra CM, Hohwy M, Griffin RG (2001) 1H–1H MAS correlation spectroscopy and distance measurements in a deuterated peptide. J Magn Reson 151(2):320–327
Schaefer J, Mckay RA, Stejskal EO (1979) Double-cross-polarization NMR of solids. J Magn Reson 34(2):443–447
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS plus: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223
Stevens TJ, Fogh RH, Boucher W, Higman VA, Eisenmenger F, Bardiaux B, van Rossum BJ, Oschkinat H, Laue ED (2011) A software framework for analysing solid-state MAS NMR data. J Biomol NMR 51(4):437–447
Suiter CL, Paramasivam S, Hou G, Sun S, Rice D, Hoch JC, Rovnyak D, Polenova T (2014) Sensitivity gains, linearity, and spectral reproducibility in nonuniformly sampled multidimensional MAS NMR spectra of high dynamic range. J Biomol NMR 59(2):57–73
Sun SJ, Butterworth AH, Paramasivam S, Yan S, Lightcap CM, Williams JC, Polenova T (2011) Resonance assignments and secondary structure analysis of dynein light chain 8 by magic-angle spinning NMR spectroscopy. Can J Chem 89(7):909–918
Sun SJ, Yan S, Guo CM, Li MY, Hoch JC, Williams JC, Polenova T (2012) A time-saving strategy for MAS NMR spectroscopy by combining nonuniform sampling and paramagnetic relaxation assisted condensed data collection. J Phys Chem B 116(46):13585–13596
Thakur RS, Kurur ND, Madhu PK (2006) Swept-frequency two-pulse phase modulation for heteronuclear dipolar decoupling in solid-state NMR. Chem Phys Lett 426(4–6):459–463
Vadlamudi RK, Bagheri-Yarmand R, Yang Z, Balasenthil S, Nguyen D, Sahin AA, den Hollander P, Kumar R (2004) Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell 5(6):575–585
Webber AL, Pell AJ, Barbet-Massin E, Knight MJ, Bertini I, Felli IC, Pierattelli R, Emsley L, Lesage A, Pintacuda G (2012) Combination of DQ and ZQ coherences for sensitive through-bond NMR correlation experiments in biosolids under ultra-fast MAS. ChemPhysChem 13(9):2405–2411
Wickramasinghe NP, Ishii Y (2006) Sensitivity enhancement, assignment, and distance measurement in 13C solid-state NMR spectroscopy for paramagnetic systems under fast magic angle spinning. J Magn Reson 181(2):233–243
Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LW, Past J, Samoson A, Ishii Y (2009) Nanomole-scale protein solid-state NMR by breaking intrinsic 1H T1 boundaries. Nat Methods 6(3):215–218
Yan S, Suiter CL, Hou GJ, Zhang HL, Polenova T (2013) Probing structure and dynamics of protein assemblies by magic angle spinning NMR spectroscopy. Acc Chem Res 46(9):2047–2058
Zhou DH, Shah G, Cormos M, Mullen C, Sandoz D, Rienstra CM (2007) Proton-detected solid-state NMR spectroscopy of fully protonated proteins at 40 kHz magic-angle spinning. J Am Chem Soc 129(38):11791–11801
Zhou DH, Nieuwkoop AJ, Berthold DA, Comellas G, Sperling LJ, Tang M, Shah GJ, Brea EJ, Lemkau LR, Rienstra CM (2012) Solid-state NMR analysis of membrane proteins and protein aggregates by proton detected spectroscopy. J Biomol NMR 54(3):291–305
Acknowledgments
This work was supported by the National Institutes of Health (NIH Grants R01GM085306, 8P30GM103519-03 from NIGMS, and 5P30RR031160-03 from NCRR). We acknowledge the support of the National Science Foundation (NSF Grant CHE0959496) for the acquisition of the 850 MHz NMR spectrometer at the University of Delaware. We thank Dr. Si Yan for preparing and packing the Cu-EDTA doped LC8 protein sample.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, C., Hou, G., Lu, X. et al. Fast magic angle spinning NMR with heteronucleus detection for resonance assignments and structural characterization of fully protonated proteins. J Biomol NMR 60, 219–229 (2014). https://doi.org/10.1007/s10858-014-9870-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-014-9870-y