Abstract
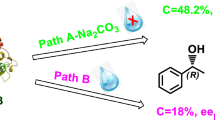
2-(Substituted phenoxy)-1-propanols, e.g. 2-(4-chlorophenoxy)-1-propanol, belonging to primary alcohols with an oxygen atom at the stereocenter, were resolved with moderate to good enantioselectivity, as judged by the value of enantiomeric ratio E (up to 27), through the enantioselective acylation with vinyl butanoate mediated by the little-known lipase from Achromobacter sp. in diisopropyl ether, after the examination of potential factors affecting the reaction such as organic solvents and acyl donors.
Similar content being viewed by others
References
Bornscheuer UT,Kazlauskas RJ (1999) Hydrolases in Organic Synthesis. Weinheim: Wiley-VCH.
Chen C-S,Fujimoto Y,Girdaukas G,Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104: 7294-7299.
Dirlam NL,Moore BS,Urban FJ (1987) Novel synthesis of the aldose reductase inhibitor sorbinil via amidoalkylation, intramolecular oxazolidin-5-one alkylation, and chymotrypsin resolution. J. Org. Chem. 52: 3587-3591.
Faber K (2000) Biotransformations in Organic Chemistry, 4th edn. Berlin: Springer-Verlag, pp. 94-123 and 344-366.
Itoh T,Uzu A,Kanda N,Takagi Y (1996) Preparation of 3-alkyl-4-hydroxy-2-butenyl acetate through highly regioselective lipasecatalyzed hydrolysis of corresponding diacetates. Tetrahedron Lett. 37: 91-92.
Kazlauskas RJ,Bornscheuer UT (1998) In: Kelly DR, ed. Biotechnology, 2nd edn., Vol. 8a, Biotransformations I. Weinheim: Wiley-VCH, pp. 37-191.
Kazlauskas RJ,Weissfloch ANE,Rappaport AT,Cuccia LA (1991) A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 56: 2656-2665.
Koskinen AMP,Klibanov AM, eds. (1996) Enzymatic Reactions in Organic Media. Glasgow: Blackie A & P.
Miyazawa T,Kurita S,Ueji S,Yamada T (2000) Resolution of mandelic acids by lipase-catalyzed transesterifications in organic media. Biocatal. Biotrans. 17: 459-473.
Miyazawa T,Yukawa T,Ueji S,Yanagihara R,Yamada T (1998) Resolution of 2-phenoxy-1-propanols by Pseudomonas sp. lipase-catalyzed highly enantioselective transesterification: influence of reaction conditions on the enantioselectivity toward primary alcohols. Biotechnol. Lett. 20: 235-238.
Okamoto T,Ueji S (1999) Drastic enhancement of the enantioselectivity of lipase-catalysed esterification in organic solvents by the addition of metal ions. J. Chem. Soc. Chem. Commun.: 939-940.
Weissfloch ANE,Kazlauskas RJ (1995) Enantiopreference of lipase from Pseudomonas cepacia toward primary alcohols. J. Org. Chem. 60: 6959-6969.
Wimmer Z,Saman D,Francke W (1994) Novel juvenoids of the 2-(4-hydroxybenzyl)cy-clohexan-1-one series. Helv. Chim. Acta 77: 502-508.
Yasufuku Y,Ueji S (1997) High temperature-induced high enantioselectivity of lipase for esterification of 2-phenoxypropionic acids in organic solvent. Bioorg. Chem. 25: 88-99.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyazawa, T., Yukawa, T., Koshiba, T. et al. Enzymatic resolution of 2-phenoxy-1-propanols through the enantioselective acylation mediated by Achromobacter sp. lipase. Biotechnology Letters 23, 1547–1550 (2001). https://doi.org/10.1023/A:1011939408925
Issue Date:
DOI: https://doi.org/10.1023/A:1011939408925